In 2021 I was shocked to discover that Australia’s Gene Technology Regulator (GTR) failed to assess the new gene technology vaccines alongside of the drug regulator, the Therapeutics Goods Administration (TGA). The new messenger RNA (mRNA) technology products were NOT looked at by the GTR but AstraZeneca’s and Janssen’s COVID-19 vaccines were!
I came to discover that the only gene technology which is assessed by Australia’s “Gene Technology” regulator are only products that contain an “organism“. This bothered me so much I wrote a substack to capture my findings, I’ve added it to this website HERE.
Even though the new 2020 mRNA gene therapy products launched by BioNTech-Pfizer and Moderna under the category “vaccine” can affect downstream human health and environmental health (as the emerging science is revealing), Australia’s government regulator (and no doubt the equivalent in other countries) which has “gene” and “technology” in it’s name, did not even look at them. You’d expect the GTR to be better positioned than the TGA with “gene technology” advisors, yet all oversight was hand-balled for mRNA to the TGA.
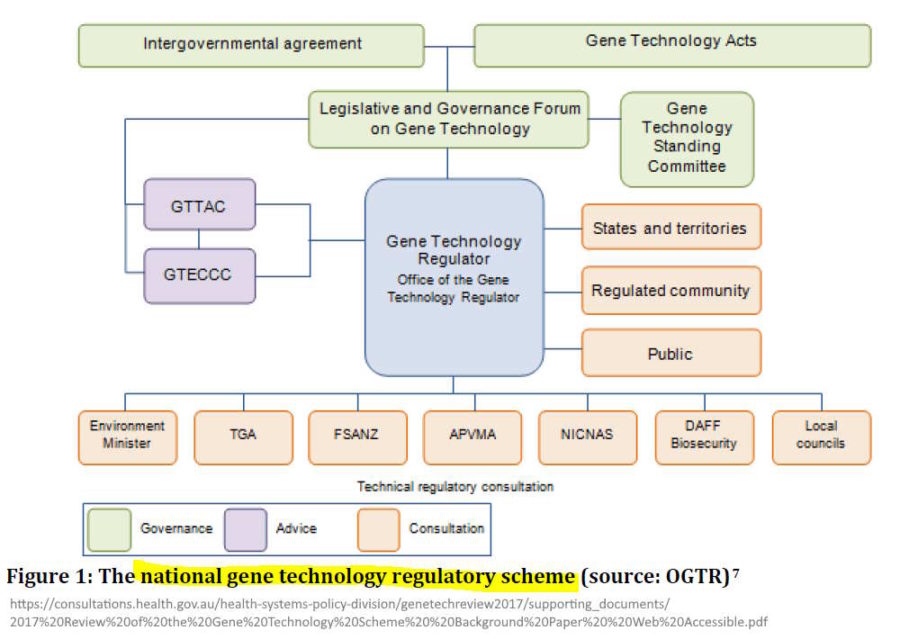
The Office of the Gene Technology Regulator (OGTR) in Australia assesses Genetically Modified Organisms (GMO), why are they not called the GMO Regulator? Their very name is deceptive, if all gene technology product do not fall under their jurisdiction. The very front page of their website is deceptive!
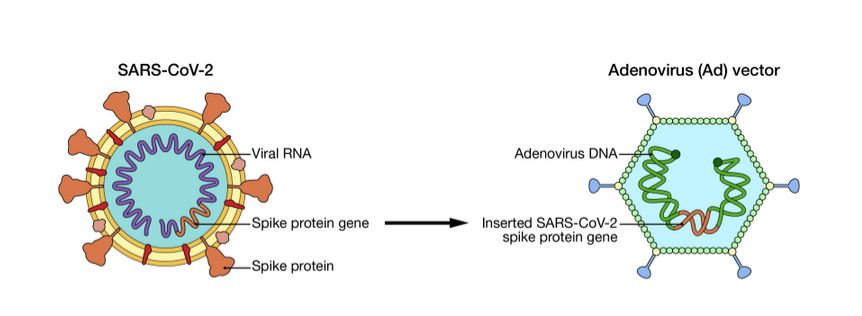
Adenovirus vectors are an ‘organism’
This diagram from the Mayo Clinic makes it clear why the adenovirus vector vaccines are assessed by the GTR, as they are an organism – genetic spike code from SARS-CoV-2 is inserted into the “replication defective” Adenovirus:
WARNING: No Fit-For Purpose Regulator!
MRNA is brand new technology, which has no established understanding of it’s human and environmental impact, has completely escaped oversight by a “fit for purpose” regulator, and more genetically modified (GM) products are coming down the line – which may end up in your body via injection or ingestions.
The highly persistent, pseudouridine ladened mRNA code, that is encapsulated with a non-natural, synthetic fatty lipid coating, to form a lipid nanoparticle could have dire consequences for off-target exposure.
But other genetic technologies on the way are Edible Vaccines, and the use of mRNA vaccines in livestock. We certainly need to pay attention to this “gene” regulator.
This page will attempt to capture some GTR history, to help understand why there are holes in their purview and if anything will be done to fix it. A place to capture notes/leads
- The Office of the Gene Technology Regulator – WEBSITE, ARCHIVES
- Annual and Quarterly Reports 2001-2018 – ARCHIVES
- Gene Technology Committees – ARCHIVES
Potential mRNA gene regulator:
Note: Upon collecting information for this page I came across a lead that maybe the National Health and Medical Research Council (NHMRC) would assess new gene technology prodcut that fall outside of the definition of “organism”. I am unable to find anything on their WEBSITE which has anything to do with assessing “mRNA”, “BioNTech” or “Moderna”. It appears all Australian regulatory oversight for the new mRNA technology fell upon the TGA and the Advisory Committee on Vaccine in 2021 and beyond
Links in reverse chronological order
2024
April 24 2024 – Dr John Campbell Channel: Genetically modified organisms an interview with Retired Barrister Julian Gillespie from Australia – WATCH, Jules on the Beach Substack – READ
March 1, 2024 – Rebekah Barnett Substack: BREAKING: Australian Federal Court throws out Covid mRNA vaccine challenge – 9 mth after injunction filed – READ
- “The Australian Courts have blocked a legal challenge over Moderna’s and Pfizer’s mRNA Covid vaccines on a technicality, stalling efforts to raise the alarm over alleged unregulated genetically modified organisms (GMOs), including high levels of DNA contamination, in the vials.”
- “Dr Fidge alleges that the mRNA Covid vaccines contain GMOs in two forms – the LNP-mod-RNA complexes, and [genetically modified] plasmid DNA contamination – for which Pfizer and Moderna never obtained the proper approvals from the Office of the Gene Technology Regulator (OGTR).”
2023
December 6, 2023 – OGTR Statement: Addressing misinformation on the regulation of mRNA vaccines – PDF, CREDIT
- The OGTR denies that the Pfizer (Comirnaty) and Moderna (Spikevax) mRNA “vaccines” are or contain GMOs, or that the products required a licence from the OGTR before being distributed in Australia, characterising such claims as “misinformation”. Handballing it to be only the TGA’s reponsibility for “safety”
- “OGTR regulates dealings (defined activities) with genetically modified organisms (GMOs) and the Pfizer and Moderna vaccines are not GMOs.”
November 21, 2023 – Press Conference Forum for Democracy in the European Parliament ( EU/FvD) led by MP Marcel De Graff – WATCH, Press Release and timeline- READ, Aussie17 – CREDIT
- Marcel de Graaff (FVD MEP) and Joachim Kuhs (AfD MEP), Willem Engel and Vibeke Manniche (doctor) discuss shocking revelations arising from the letter from the European Medicines Agency (EMA). This letter came in response to the request from Marcel de Graaff and Joachim Kuhs to immediately suspend the licenses of Covid-19 vaccines.
- “First of all, the EMA explicitly states that it has exclusively allowed the corona vaccines on the market for individual immunizations and absolutely not for the control of infection and absolutely not for preventing or reducing infections. And this is devastating for governments that have gone full circle with the message that you are doing it for someone else.”
- “…we are still fighting with a gigantic so-called unexplained excessive mortality. …The government knew that the vaccines would not protect against the spread of the virus, but did not share this information with the citizens….
- “To be very clear, the mRNA is non-human and part of a GMO. Moreover, the recent publications on plasmid DNA contamination in these mRNA injection fluids proves beyond doubt that we are dealing with a GMO product. Regardless of the attempts to redefine the term GMO, following an EU court ruling from 2018, any innovation containing or derived from the use of genetic engineering techniques is to be considered the same as a GMO and subject to the same regulatory approval system. Now, integration of bacterial or viral genetic code into the human genome has been associated with a higher risk of cancer.”
November 14, 2023 – Julian Gillespie Substack: Australian Criminal Brief Against Pfizer & Moderna .. Update presented to Attorney-General -Pfizer & Moderna’s C19 drugs were always GMOs – READ
- Dr Julie Sladden “… other manufacturers/sponsors who have wanted to import and transport GMOs into Australia have always been required to apply for GMO licenses” so why were Moderna and Pfizer-BioNTech exempt? Pfizer met with Bhula in 2020.
- The SV40 promoter sequence is now known to be able to enter the nucleus and bind to p53 hereby diminishing the capacity of the Guardian of the Genome to protect Pfizer recipients from cancer – Also READ
October 29, 2023 – Julian Gillespie Substack: Australian Gene Technology Regulator, Dr Raj Bhula .. admits modRNA products are GMOs .. then tells everyone a furphy – READ
- February 16, 2023 – Dr Bhula appeared before the Australian Senate’s Community Affairs Legislation Committee and stated “The mRNA Covid-19 vaccines did not involve any step of genetic modification.“!
October 26, 2023 – Julian Gillespie Substack: The Covid Lawsuit Australia Has To Have – Australian politicians & political parties will Never conduct truly critical Inquiries or properly empower any Covid Royal Commission to do so .. to do so would reveal their legal liability. – READ
- “Our team [Lawyers Katie Ashby-Koppens, Peter Fam and Julan Gillespie] recently launched new proceedings this time directly at Pfizer and Moderna, to show their C19 products always met Australian legal definitions for being properly deemed GMOs, (genetically modified organisms – both the modRNA and the synthetic DNA contamination), therefore both companies should have applied for GMO licenses from the Office of the Gene Technology Regulator (OGTR).”
- “…the final act of genetic manufacture does indeed occur in Australia” inside human cells – states an anonymous Australian Professor of science and medicine, “What OGTR are proposing is thereby a nonsense. If they are redefining the Act in this way then it means we can create all manner of new genetic organisms and providing we do this in vivo [inside human cells] then it is now outside the purview of the OGTR”
October 26, 2023 – Senator Rennick: Australian Senate Estimates hearings with the Office of the Gene Technology Regulator (OGTR) – Pfizer confirms gene therapy & the Office of Gene Tech are still in denial- READ, WATCH, Transcript – PDF, Arkmedic “they lied”… – READ, JikkyLeaks – EXCERPT
If in deed the mRNA was being manufactured here [in Australia] and its correct that gene technology was used, the modification of the mRNA. Then under the Gene Technology Act an approval would have been required for that manufacturing step…the Gene Technology Act doesn’t reach into other countries”
Dr Raj Bhula – Gene Technology Regulator
- The mRNA gene technology products transfect cells of Australian citizens – meaning the last manufacturing step for the “antigen protein” is following the product entering human cells. Dr Bhula stated under oath “No, I disagree with that”
- “Transfection is the process of deliberately introducing naked or purified nucleic acids into eukaryotic cells” – WIKI
- Getting inside the cells is how Australian’s were told the products would work ..the government just didn’t use the technical term “transfection”. It’s even mentioned in the manufacturers pre-clinical evaluation report
October 10, 2023 – CMN News Substack: Can Pfizer and Moderna COVID Vaccines be deemed Genetically Modified Organisms? And…Are they contaminated with Foreign DNA? – LEGAL ACTIONS LAUNCHED – READ, CREDIT
- Case File Number: VID510/2023 Julian Fidge v. Pfizer Australia Pty Ltd & Anor
- Case Summary: It is alleged the Covid-19 vaccines (both the monovalent and bivalent products) produced by the Respondents Pfizer and Moderna satisfy the Australian legal definitions for being deemed Genetically Modified Organisms, pursuant to section 10 of the Gene Technology Act.”
October 9, 2023 – WCH Symposium: Urgent Expert Hearing on Reports of DNA Contamination in mRNA Vaccines – ALL VIDEOS (many more speakers)
- Australian Attorney Katie Ashby-Koppens: The Australian GMO Case Against Pfizer and Moderna – WATCH
“The allegations are that the Covid-19 mRNA vaccines are genetically modified organisms by way of definition under the Gene Technology Act in Australia, as they are capable of transferring genetic material.”
Katie Ashby-Koppens
September 5, 2023 – Senator Gerard Rennick: The Health Minister must investigate Fauci correspondence with Sydney Uni – re Eddy Holmes – READ, The Letter – READ, ARCHIVE, CREDIT
- Sen Rennick asks Dept Healths’ OGTR to investigate Eddy Holmes involvement in the virus origins coverup. Dept Health wrote back praising Holmes! – READ
February 16, 2023 – Senator Rennick: Australian Senate’s Community Affairs Legislation Committee: mRNA jabs use genetic engineering yet the TGA didn’t consult with the Gene Tech Regulator – WATCH, CREDIT
- Australian Government genetechnology.gov.au : Gene technology and human health – READ, About – ARCHIVE
- “An example of GMOs as medicines is gene therapy….At its simplest, gene therapy involves inserting DNA or RNA into cells to treat or prevent diseases that are not treatable with medicines.”
2022
January 10, 2022 – TGA – New industry catagory ADDED called Advanced Therapies, under which gene therapies suddenly appears! – ARCHIVE, ARCHIVE, READ,
- Any biological or prescription medicine that involves genetic modification must also be approved by the Office of the Gene Technology Regulator (OGTR) [WOW! COVID-19 mRNA vaccines skipped that step in 2020!]
- “We regulate therapies that involve in-vivo genetic manipulation of human cells as prescription medicines under section 23 of the Act. This includes small silencing RNAs, CRISPR and other gene editing technologies, and gene therapies administered by vectors.”
2021
June 25, 2021 – OGTR: DIR 184 – Clinical trial with a genetically modified human adenovirus COVID-19 vaccine – Avance Clinical Pty Ltd is conducting a clinical trial – to assess intranasal administration of a GM vaccine for COVID-19 – READ, ARCHIVE, application received March 16, 2021
- Notification of decision on application DIR 184 from Avance Clinical Pty Ltd for a clinical trial with a genetically modified human adenovirus COVID-19 vaccine – PDF
Who is Avance Clinical?:
- Avance Clinical Contracted for Atossa Therapeutics AT-301 Nasal Spray Clinical Study, Atossa’s Second COVID-19 Therapeutic Development Program – READ, ARCHIVE, Atossa nasal spray – READ, first archived 2020!- ARCHIVE
- Avance Clinical Congratulates Client Tetherex Pharmaceuticals on Initiation of Dosing in a Phase 1 Clinical Study Using a Novel Single-Cycle Adenovirus Vaccine Strategy in Australia – READ, ARCHIVE
- Avance Clinical is accredited as a gene technology CRO under the Office of the Gene Technology Regulator (OGTR). – REF
April 19, 2021 – Office of the Gene Technology Regulator (OGTR) Australia: DIR 182 – Commercial supply of a genetically modified COVID-19 vaccine – Janssen-Cilag Pty Ltd [Johnson & Johnson COVID-19 vaccine], Adenovirus, Vaccine – altered antigen expression; Vaccine – replication incompetent – READ, ARCHIVE, Date application received December 2, 2020
- Licence decision – notification – PDF
- Licence decision – Q&A – PDF
- Risk assessment and risk management plan – summary – PDF, Full – PDF
- Licence Condition – PDF
February 8, 2021 – Office of the Gene Technology Regulator (OGTR) Australia: DIR 180 Commercial supply of a genetically modified COVID-19 vaccine – AstraZeneca COVID-19 vaccine – READ, ARCHIVE, Vaccine – altered antigen expression; Vaccine – replication incompetent – Application received Dec 7, 2020
- The Gene Technology Regulator (the Regulator) has decided to issue a licence for application (DIR 180) for the import, transport, storage and disposal of a genetically modified (GM) COVID-19 vaccine, as part of its commercial supply as a human vaccine.
- Licence decision – notification – PDF
- Before the GM vaccine can be used, AstraZeneca must also obtain regulatory approval from the Therapeutic Goods Administration (TGA).
- “The risk assessment concluded that risks to the health and safety of people or the environment from the proposed supply, either in the short or long term, are negligible. The current assessment focused on risks posed to people other than the intended vaccine recipient and to the environment,…”
2018
October 31, 2018 – Annual and Quarterly Reports under the Gene Technology Act 2000 – links to reports from 2001-2018 – ARCHIVE
- The Reports provide a detailed description of the OGTR’s activities during each quarter and financial year.
- Includes Genetic Manipulation Advisory Committee (GMAC) Annual Report 1998 & 1999
2017
September 15, 2017 – OGTR Newsletter release their “first Newsletter” – PDF, SOURCE
July 2017 – The Legislative and Governance Forum on Gene Technology – 2017 Review of the National Gene Technology Regulatory Scheme – Background Paper – PDF, SOURCE
- “The Review of the Scheme by the Forum is independent of the Gene Technology Regulator (the Regulator) and the Office of the Gene Technology Regulator (OGTR), and is separate to the Review of the Gene Technology Regulations 2001 (GT Regulations) currently being undertaken by the Regulator.’
- What is Gene Technology and Genetically Modified Organisms? The publication answers
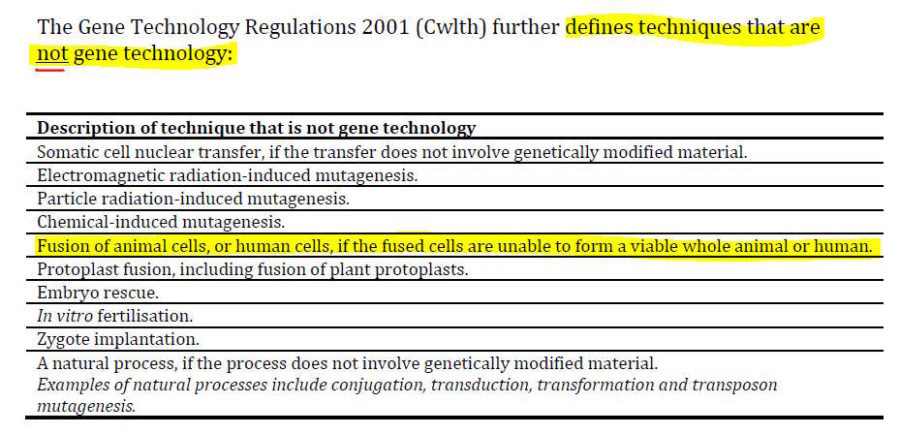
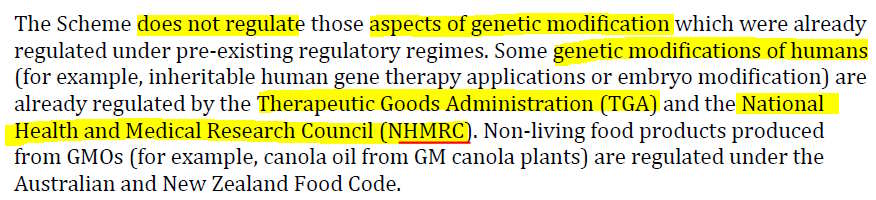
- From this it appears that the National Health and Medical Research Council (NHMRC) is where we will find mRNA technology oversight – besides the already known TGA which forced the products through the March 2018 Provisional Registration pathway
January 2017 – TGA Advisory Committee on Vaccines (ACV) was established – REF This is the sole regulatory body that assessed the mRNA vaccines as far as I can determine.
- ACV in Dec 2020 had no committee members that were expert in gene technology – MEMBERS, READ
- Therapeutic Goods Regulations 1990 – August 2020 – PDF, ALL (try and find the definition of “vaccine”!)
This is the sole body that assessed the new gene technology mRNA Vaccines

So the regulations asked to establish an Advisory Committee on Vaccines, but they did not define what is a vaccine, or who determines if a product can be designated “vaccine”!
2016
October 17, 2016 – OGTR: 2016-17 Technical Review of the Gene Technology Regulations 2001 begins with submissions – ARCHIVE, all ARCHIVES
2002
January 25, 2002- Commonwealth Department of Health and Ageing | Office of the Gene Technology Regulator: Quarterly Report of the Gene Technology Regulator – for the period 1 July to 30 September 2001 – PDF, SOURCE
- Public were consulted on going from the voluntary system to legislative framework – Public consultation on the draft framework began early in September 2001: Risk Analysis Framework for Licence Applications to the Office of the Gene Technology Regulator
2001
September 11, 2001 – Department of Health: The Gene Technology Agreement was signed: The Gene Technology Agreement (GTA) is an inter-governmental agreement which sets out the understanding between Commonwealth, State and Territory governments regarding the establishment of a nationally consistent regulatory system for gene technology – it was reconfirmed in 2008 – ARCHIVE, READ, 2008 – ARCHIVE
- TGA: The Gene Technology Ministerial Council was established by the intergovernmental Gene Technology Agreement 2001 – Council – ARCHIVE, Agreement – ARCHIVE, PDF
- Sept 5, 2023 – Policy principle to recognise genetically modified (GM) /Non-GM designated areas – final regulatory impact statement – ARCHIVE, Gene Technology (Recognition of Designated Areas) Principle 2003 ARCHIVE
June 21, 2001 – The Gene Technology Act 2000 (the Act) came into effect on 21 June 2001 establishing a nationally consistent regulatory system for gene technology which replaced a system of voluntary controls – REF, REF2
2000
December 8, 2000 – Department of Health: The passage of Gene Technology Act 2000 through the Commonwealth Parliament on December 8, 2000 and Royal Assent on December 21, 2000 – PDF
- GTA is a national scheme for the regulation of genetically modified organisms in Australia, in order to protect the health and safety of Australians and the Australian environment by identifying risks posed by or as a result of gene technology, and to manage those risks by regulating certain dealings with genetically modified organisms. – READ (see June 21, 2021 above)
- Interim Office of the Gene Technology Regulator was established as a Branch of the Therapeutic Goods Administration in May 1999 – REF
June 22, 2000 – Interim Office of the GeneTechnology Regulator (IOGTR) release their first quarterly report with Gene Technology Bill 2000 – PDF, SOURCE
- “Developing a new national regulatory system for genetically modified organisms (GMOs), [yet they call it gene technology!] including the Gene Technology Bill 2000, the Gene Technology (Consequential Amendments) Bill 2000, and the Gene Technology (Licence Charges) Bill 2000.” Tasmania elects to opt-out!
- It’s purpose to “protect the health and safety of people, and to protect the environment, by identifying risks posed by or as a result of gene technology, and by managing those risks through regulating certain dealings with GMOs.”
- Gene Technology Bill 2000 being introduced into Parliament on 22 June 2000, the IOGTR developed and released a draft of the corresponding Gene Technology Regulations 2000 –REF
July 14, 2000 – Australian Children’s Hospital at Westmead and Children’s Medical Research Institute joint initiative establish the Gene Therapy Research Unit – ARCHIVE, ARCHIVE, Challenges ARCHIVE
- Gene Therapy, or “the use of genes as medicine” is a paradigm with immense but largely unrealised potential – REF
- Gene Therapy, which can be defined as “an attempt to cure or favourably modify the natural history of a disease process by altering one or more genetic events within a defined target cell population“.
- A major obstacle to the implementation of Gene Therapy clinical trials in Australia is the problem of access to clinical grade gene delivery formulations (vector)” – REF
1999
1999 – Genetic Manipulation Advisory Committee (GMAC) Annual Report 1998-99 – PDF, SOURCE
1998
June 30, 1998- Genetic Manipulation Advisory Committee (GMAC) Annual Report 1997-98 – PDF, SOURCE
- “GMAC was formerly the Recombinant DNA Monitoring Committee (RDMC), within the Industry, Technology and Commerce portfolio, from 1981 until 1987. From 1988 to March 1996, GMAC was within the Administrative Services portfolio.”
April 1998 – Guidelines for Activities with the Potential for Unintended Release of Genetically Manipulated Organisms – REF
1997
October 30,1997 – Australian Government announced a national regulatory framework for genetic manipulation work (‘gene technology’) would be established. The “new legislation to provide some statutory control of gene technology research and to provide statutory coverage of general releases of genetically modified organisms that are not covered by existing bodies.” – [Pg 43]
1996
March 11, 1996 – GMAC was transferred from Administrative Services in the Finance portfolio back to the Industry, Science and Tourism portfolio which it had prior to July 1988 – [Pg 42]
1994
1994 – Gene Therapy Committee was established by the National Health and Medical Research Council (NH&MRC) to assess proposals for human gene therapy – [Pg 42]
- Gene therapy proposals are submitted directly to the Gene Therapy Committee (changed to Gene Therapy
Research Advisory Panel), rather than to GMAC. - “Liaison between GMAC and the Gene Therapy Committee is maintained by cross-membership between the Committees; two members of GMAC’s Scientific Subcommittee are members of the Gene Therapy Committee.”
- [This is the first clue I’ve found that maybe Australia does have a regulatory body to assess mRNA technology!]
1990
June 12,1990 – The Minister for Industry, Technology and Commerce [who previously was responsible for GMO oversight] wrote to the House of Representatives Standing Committee on Industry, Science and Technology proposing an inquiry into the issues arising from, and the regulation of, genetically modified organisms. – [Pg 41]
- The Committee’s report, Genetic Manipulation: the Threat or the Glory?, was tabled in February 1992 – Report – READ Legislation was proposed.
- Ch2: Existing system of regulation
- Ch3: Existing and potential benefits
- Ch4: Philosophical/ethical/social issues – PDF – see Germ cell gene therapy & Somatic pg 90
- Ch5: Environmental issues
- Ch6: Human health issues – PDF, Regulation of pharmaceuticals pg 210
- Ch7: Legal issues

1988
December 1988 – The first Genetic Manipulation Advisory Committee (GMAC) meeting took place in Canberra under the new Minister for Administrative Services. The committee was created in Sept. 1987 – REF
1987
September 1987 – Genetic Manipulation Advisory Committee (GMAC) was created, announced by the Minister for Industry, Technology and Commerce to replace the Recombinant DNA Monitoring Committee (RDMC), “with somewhat wider terms of reference” – REF
- “Responsibility for GMAC was transferred to the Minister for Administrative Services in July 1988. In August 1988, members were appointed to GMAC by the then Minister for Administrative Services and the first GMAC meeting took place in Canberra in December 1988.”
1986
1986 – RDMC: Monitoring Recombinant DNA Technology: A Five Year Review – REF, READ, NSW library – HERE A report to the Minister for Industry, Technology and Commerce [Pg 41] – Acknowledged that “novel systems were constantly being introduced…”
- This report “concluded that, since there were some areas in which possible hazards could be seen and novel systems were constantly being introduced, the technology should continue to be monitored to ensure that appropriate safety standards and practices were adopted.
- The review also concluded that the non-statutory monitoring system had been effective and was likely to remain so for at least the next five years.”
1981
October 1981 – Recombinant DNA Monitoring Committee (RDMC) was established out of the ASCORD guidelines. The committee resided within the Aust. Govt. Department of Science: Industry, Technology and Commerce portfolio – REF
- This committee produced three sets of guidelines: [Pg 41]
- for small scale contained work (volumes less than 10 litres),
- large scale contained work (volumes greater than 10 litres, usually industrial) and for
- planned releases of live organisms to the environment
1975
1975 – Investigation into the safety of recombinant DNA techniques – Australian guidelines set by ASCORD – REF, Dr Malone on Asilomar Conference on Recombinant DNA – READ
- “In the early 1970s, when the technology was being developed, some scientists became concerned that it might be possible to create hazardous microorganisms using recombinant DNA techniques. The scientists themselves called for an investigation of the safety of the technique. Molecular biologists from around the world, including two from Australia, met for this purpose at Asilomar in California in 1975. The outcome of the Asilomar meeting was that scientists decided to continue recombinant DNA research using precautions to contain any possible hazards.”
- “In response to this conclusion, the Australian Academy of Science set up a Committee on Recombinant DNA (ASCORD) which drew up the first Australian guidelines for these techniques in 1975″ [Pg 41]