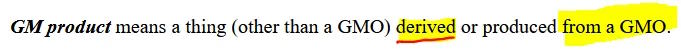This post was first written for Totalilty of Evidence substack June 20, 2023 – HERE
Australia’s Gene Technology Regulator did NOT assess the new gene technology vaccines
The mRNA COVID-19 vaccines were 100% exempt of oversight by the Gene Technology Regulator because they did not fit the definition, but guess what AstraZeneca and J&J did.
In early 2021 I conducted a little research into Australia’s Gene Technology Act 2000 and it’s respective regulations the Gene Technology Regulations 2001 [1] in relation to assessing the new gene technology vaccines and what I found totally shocked me. I’ve been meaning to document this for some time, so here goes.
When I went to the Office of the Gene Technology Regulator’s (OGTR) website to look for their assessment documents for the brand new messenger RNA (mRNA) products, which their manufactures submitted to the TGA under the category “vaccine,” I could not find them.
Upon further investigation I realised these genetic vaccines were 100% exempt of assessment and oversight by Australia’s Gene Technology Regulator!
Wait what?
How could a new product that is based on gene technology be excluded from assessment by the very regulator that has “gene technology” in it’s name?
Both BioNTech-Pfizer and Moderna’s mRNA vaccines evaded assessment by the OGTR and went straight to the Therapeutic Goods Administrator (TGA).
But does the TGA even have the expertise to assess a genetic technology product and all it’s potential consequences to the environment? Maybe we’ll get back to that!
AstraZeneca & J& J were assessed
So I did find documents relating to both AstraZeneca’s new technology COVID-19 “vaccine” [DIR 180 [2, 3, 4] and Johnson & Johnson’s COVID-19 vaccine [DIR 182 [5]] at OGTR, in which I came to realised that the Gene Technology Regulator ONLY assess products that are Genetically Modified Organisms (GMOs). Both A/Z and J&J have a virus vector – “an organism”. It is not clear if they actually assessed the genetic code for the spike protein.
The OGTR ONLY assess“ORGANISMS”!!!
It took a bit but after much digging, I came to the conclusion that the OGTR only assesses Genetically Modified Organisms (GMO) with emphais of “organism”.
But that is not clear from the objective of the Act which states:
“The object of this Act is to protect the health and safety of people, and to protect the environment, by identifying risks posed by or as a result of gene technology and by managing those risks through regulation certain dealings with GMO’s.”

I’m not a lawyer, but I see the objective clearly has an “and” in relation to GMOs, it doesn’t state that “gene technology” is exclusive to “organisms”. Remember this Act was written in 2000 a time when biotechnology and nanotechnology were beginning to take off. (See more on that in the Totality of Evidence – TIMELINE.)
Protect the Environment
So if this regulator is set up to protect the enviroment from gene technology products, but mRNA vaccines are exempt of their oversight, who’s job is it then to assess environmental impact?
How much of the synthetic, pseudouridine-saturated mRNA genetic codes, the lipid nanoparticles (LNP) or the actual spike protein is getting excreted from the human body, down the toilet and into our waterways? Did anyone even look at this? What is the downstream consequences of this to the “health and safety of people” plus the impact on the environment. (That’s before we even look at them injecting mRNA into our livestock)
Given that this protein is likely constructed in a laboratory, does that not pose an even greater threat to the environment? At the very least did some regulator look at this?
So if it is not the Gene Technology Regulator’s job, then did the Therapeutic Goods Administrator (TGA) perform such an assessment? I don’t know the answer, but these are the questions I have.
Why is this bureaucratic department not called the “GMO Regulator”?
If GMO’s are all the Gene Technology Regulator assesses, would it not be more accurate to call them the GMO Regulator! On their website they certainly don’t make that clear, look at their front page, they assess “risks posed by gene technology”!
About the Regulator?
On the OGTR About page (Jan 2021) it states that one person is the Gene Technology Regulatory (GTR), who’s position lasts for 5 years, and was due to expire July 2021:
Dr [Rajumati] Raj Bhula is the Gene Technology Regulator, appointed for a period of five years commencing 18 July 2016. . She is responsible for administering the national regulatory system for gene technology as set out in the Act. The OGTR staff are part of the Department of Health.
It appears Dr Bhula was reappointed [6] again for another 5 years, ending in 2026 [7].
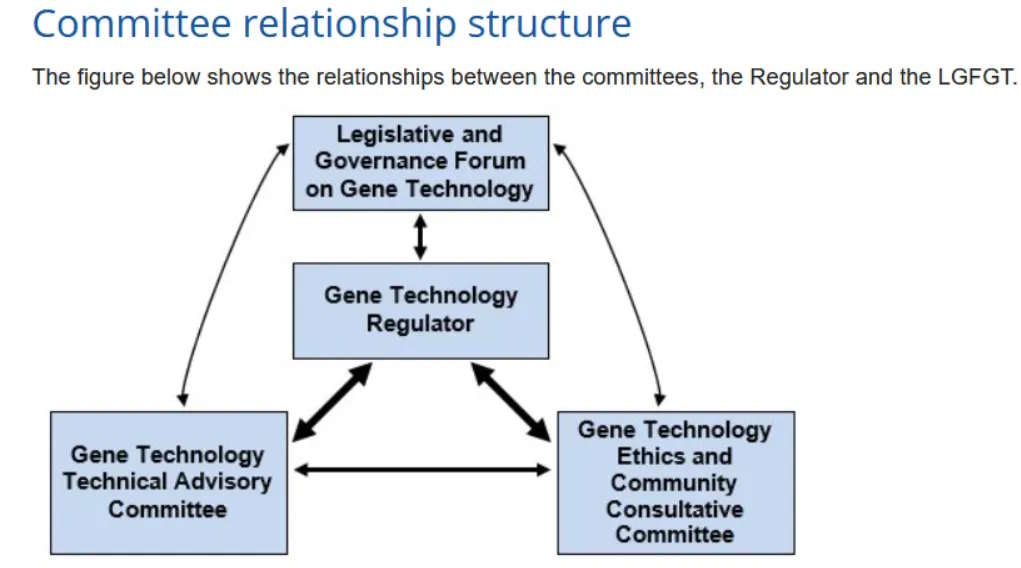
So like most regulators (so I am learning) they have advisory bodies to help guide their decisions. The Gene Technology Regulator has an advisory committee, GTTAC [8] and an ethics committee, GTECCC [9, 10].
It is stated that the Regulator seeks advice from the Gene Technology Technical Advisory Committee (GTTAC) on licence applications to work with genetically modified organisms (GMOs) and on Risk Assessment and Risk Management Plans (RARMPs) prepared for applications
Intentional Release (DIR) of a GMO into the environment can involve a limited and controlled release (clinical trial or field trial) or a commercial (general) release.
This regulator is not set up to handle anything but GMOs!
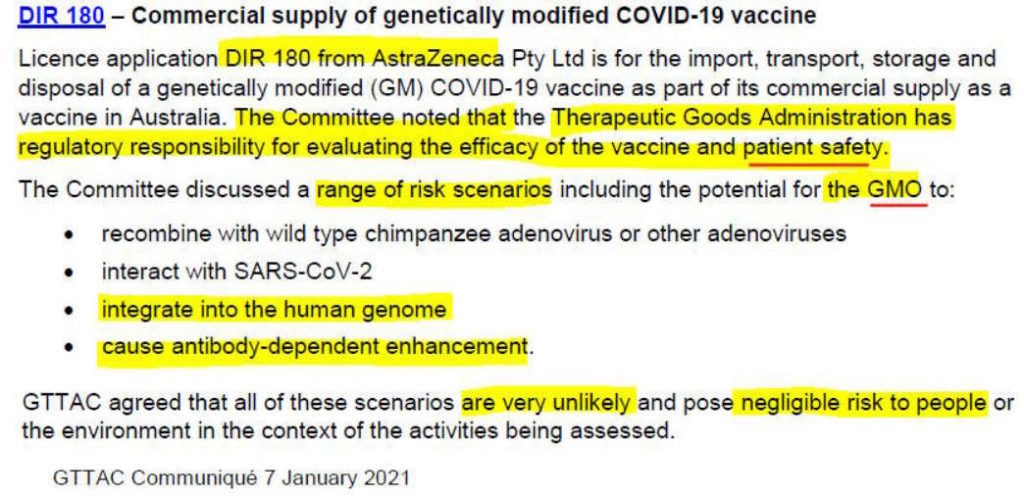
Lets look at their assessment of AstraZeneca’s vector
In the case of AstraZeneca’s vaccine on January 7, 2021, the GTTAC determined [11] the product posed “negligible risk to people or the environment”. Based on what data it had at the time, especially for whether the DNA could “integrated into the human genome” or cause Antibody Dependent Enhancement (ADE)!
So interestingly if they look at ADE and gene integration for vector vaccines…they don’t just handball human safety assessment off to the TGA. That’s interesting.
I’m unclear if the assessment was only made on the virus vector, or whether they also considered the SARS-CoV-2 spike protein DNA code?
So lets look at some definitions in The Act
The Gene Technology Act 2000 defines gene technology as:

So gene technology is any technique for the modification of genes OR genetic material. So the gene tech process to make the product?
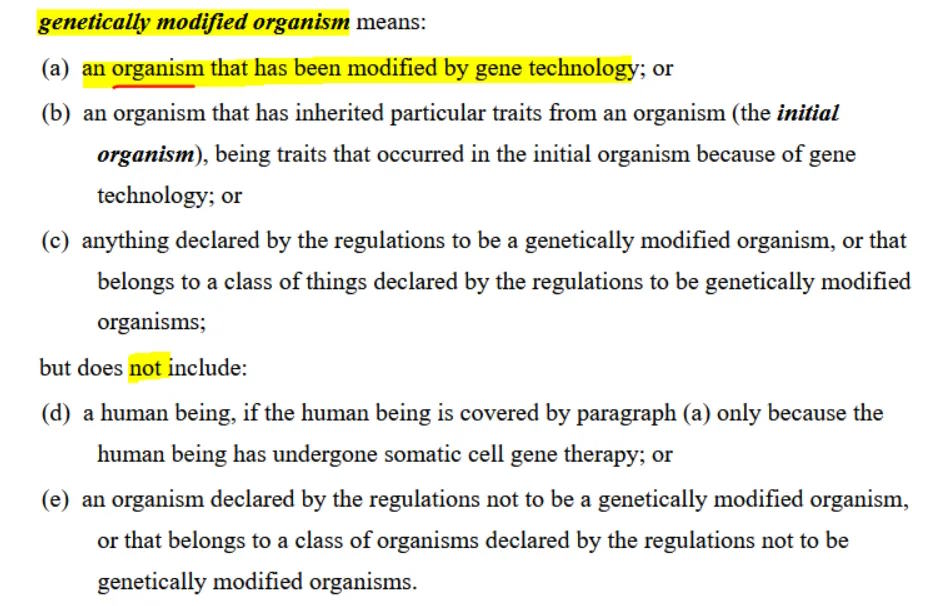
The definition of genetically modified organism (GMO):
So how do they define “organism”? Any biological entity that is capable of transferring genetic material, which meets one of three criteria, a, b or c.
“Any biological entity”:
The Act does not define “biological entity”, infact a word search show entity is only used once. But by definition is means the existance of something.
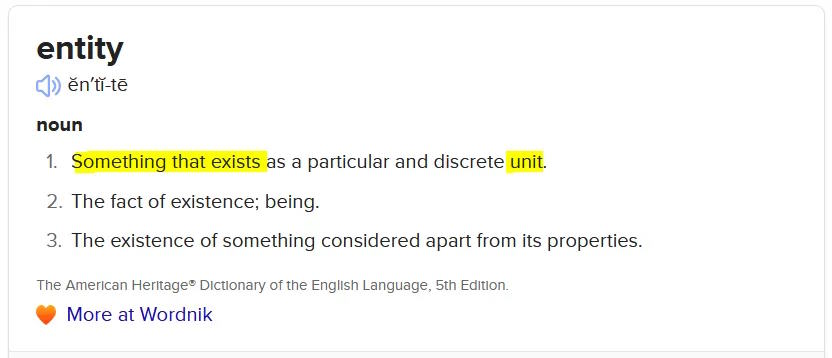
I’m wondering if there is a legal argument that mRNA COVID-19 products could fulfil the criteria for an “organism”? The existance of something which is biologically active which fulfils criteria (a), (b) OR (c):
- A synthetic Lipid Nanoparticle (LNP) is biologically active, in that it fuses with cell walls to allow the transfer of genetic material into a cell – The LNP encapsulates mRNA which certainly assists it’s biological activity – waiting for fertile grond like a seed.
- Could mRNA encapsulated by LNP be a “biological entity”? It is “capable of transferring genetic material”, the mRNA code, from the “entity” into a cells cytoplasm, to then “reproduce” over and over the genetically modified protein it is designed to make – just like A/Z and J&J’s product in an overall sense, it does not reproduce itself, but what it is designed to make.
- The mRNA genetic sequence is “capable of success” in it’s purpose, which makes it “viable”. It is capable of hijacking ANY cell (thanks to the non-selective nature of the LNP) and forcing that cell to manufacture spike protein – so the “biological entity” is definitely “viable”, that alone fulfils (a).
- mRNA is possibly more viable than the virus vectors, due to the pseudouridine insertions, making it capable of reproducing proteins, possiblyd for at least 60 days maybe longer!
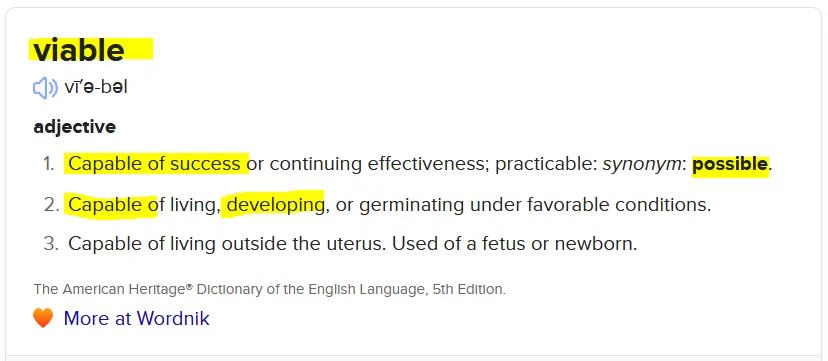
I think one could argue that the LNP plus mRNA, the components of the Moderna and Pfizer-BioNTech COVID-19 vaccines do fulfil the definition of “organism”?
mRNA vaccine “entity” is no more “alive” than a virus, or vaccine virus vector. If the latter meets the definition then why not LNP/mRNA?
One more definition
A Genetically Modified (GM) product, is defined in the legislation as “a thing (other than a GMO) derived or produced from a GMO”.
We could look at this two ways. The product could be the genetically modified “spike protein” produced in the cell as a result of the mRNA GMO!
OR maybe we take it back a step, and consider the manufacturing process uses a GMO to produce the bulk mRNA.
Are you still with me?
To mass produce the mRNA, Pfizer (possibly Moderna) used genetically modified E.coli bacteria, with whith the spike antigen is “cloned in an E.coli plasmid vector” [12, 13]. The E. coli are mass produces mRNA – and DNA contaminants…something else the Gene Regulatory should be interested in! This is definitely a GMO step.
The harvested mRNA is then, “purified” and encapsulated by the LNP, It could be argued that the mRNA in this view is the GM product derived from GMO E.coli.
Maybe if we explain it like a plant:
The LNP encapsulated, genetically modified mRNA entity acts like “seeds”, which when it is “planted” in the human body, via an injection into the deltoid muscle, the seeds make their way throughout the “fertile garden” eventually making their way into the “soil” which is ANY cell. Inside the cell the mRNA forces the production “the growth stage” of a genetically modified product, the spike protein. It reproduces spike protein over and over – for as long as it is programmed to do.
Inadequate oversight in Australia
In my opinion, these mRNA product should have undergone oversight for by Australia’s Gene Technology Regulator!
The more I look the more I realise that currently Australia does NOT currently have a regulator in place, or certainly not cearly definded, to adequately assess new mRNA gene technology products, products that don’t easily fit the definition of “organism”, but which could be argued into that category.
Maybe mRNA should just self-identify as an organism! At least then they wouldn’t be left out!
Currently the TGA is solely responsible for ALL mRNA oversight – including environmental impact!

In the screenshot above for AstraZeneca the GTTAC committee state the TGA “has regulatory responsibility for evaluating the efficacy of the vaccine and patient safety.”
Why then did GTTAC look at ADE? Isn’t that the TGA’s job, as it is relavent to patient safety? Clearly there is some overlap for a product that makes it to the GTR’s desk.
If it can’t be argued that these mRNA products fulfil the definition of “organism” then the TGA are stuck assessing everything about these new gene technology products that the pharma companies have submitted under the product category of “vaccine”!
TGA Advisory Committee on Vaccines
Again, do the TGA have adequate expertise? The TGA Advisory Committee on Vaccines (ACV)[14] do all the assessment for vaccines.
If a vaccine has a GMO then the OGTR get involved:
The TGA is required to inform the Office of Gene Technology Regulator about applications for the supply of therapeutic goods that contain GMOs [15]
The GTR should argue that mRNA techonolgy products fulfil “organism” status….AT THE VERY LEAST WE SHOULD UPDATE THIS LEGISTRATION TO INCLUDE ALL GENE TECHNOLOGY PRODUCTS SUCH AS MRNA!!!
Compare the skills of the TGA vs OGTR’s advisory committee members:
- TGA vaccine advisory committee from late 2020 – HERE
- OGTR Gene Technology Technical Advisory Committee – HERE
I’d say its pretty clear the TGA are not experts in assessing genetic technology!
Have we grandfathered in mRNA oversight to the TGA BECAUSE of an “emergency”?
It seems obvious to me that mRNA gene-technology products labelled “vaccines” were forced into our country under the guise of an “emergency” without an appropriate regulatory system to assess their new and unique properties and downstream consequences.
They don’t easliy fit any existing regulation, that I am aware of.
Lets go back to June 21, 2001 when the Gene Technology Regulator Scheme was set up. It is written that regulation was :
Australia’s National Gene Technology Regulatory Scheme which regulates gene technology, arose from the need to provide regulatory coverage for genetically modified organisms (GMOs) and genetically modified (GM) products not regulated under existing regulatory schemes. [16]
As there was no existing means of regulating this new technology, the GTR was established. It has failed to capture the 2020 emerging genetic therapy technology.
Messenger RNA is a technology that was forced on Australia under an “emergency” and as such slipped into a March 2018 TGA clause called “PROVISIONAL REGISTRATION”.
Without the Theraputic Good Administration Act’s Provisional Registration provision, Australia WOULD NOT have had a pathway with which to FAST TRACK “approval” of mRNA vaccines in Australia.
In short, Australia has NO suitable regulator in place to cater to regulating the environmental consequences and broader human impact of these new form of gene technology products.
- With long-lasting pseudouridine-mRNA together with the LNP being transported to ANY cell in the body, it is highly probalby that vaccine byproducts are being excreted into the wastewater and the environment – at what cost.
- Who knows what other avenues of contamination we’ll discover
Support the efforts at Totality of Evidence
If you find the content at Totality of Evidence useful for yourself or to help awaken your family and friends, please consider becoming a paid subscriber so I can continue to add to the historical record.
Otherwise share the website on social media and subscribe to my substack so you get my next stack in your inbox!