The World Health Organization (WHO) is a special division of the United Nations it’s constitution came into force on April 7, 1948. It’s HQ is located in Geneva, Switzerland. The WHO is governed by 194 Member States (countries who have signed onto the WHO constitution) who meet annually at the World Health Assembly. The main tasks of the World Health Assembly (WHA) are to approve the WHO programme and the budget for the following biennium and to decide major policy questions.” [1, 2, 3]
The WHO’s objective, as set out in its Constitution, is the attainment by all peoples of the highest possible level of health
Health is… a state of complete physical, mental and social well-being and not merely the absence of disease or infirmity.
As is defined in WHO’s Constitution
The World Health Organisation (WHO) believes “vaccines” and “immunization” for everyone is the backbone of of the most cost effective “health” for all. The “recommendations” of the WHO profoundly influences global immunization programs. This is an attempt to understand the history behind the vaccine agenda.
The purpose of this page
This page will capture the web of WHO’s influential programs, frameworks, working groups and agendas relating to “vaccines” and “immunisation”. Every 10 years the World Health Organisation (WHO) plans a new agenda to focus their efforts, usually building on, or growing out of, the previous agenda that encapsulates vaccination.
The WHO brings together a unified and philanthropically funded voice of vaccination policy which ALWAYS starts with targeting the poor and developing nations and has now morphed into the “leave no one behind” under the banner of “equity” which is part of the Agenda 2030 Sustainable Development Goals (SDG). 14 of the 17 SDG’s are measured by vaccination coverage!
Many philanthropic non-government organisations (NGOs) or foundations donate to the WHO or support their efforts in parallel, no doubt profiting from the knowledge of what is pushed.
This page is a place for me to bookmark historical documents to piece together the vaccine agenda which grew from a $6 billion market in 2002 to $78.27 billion in 2023 and is well estimated to grow to $122.27 billion by 2030.
History of the World Health Organization (ARCHIVE)
- The First 10 years (1948-1957) – READ
- The Second 10 years (1959- 1967) – READ
- The Third 10 years (1968 – 1977) – READ
- WHO “The History of Vaccination” – @2000,
Links in reverse chronological order
This content will continuously be updated, it will never be complete as WHO changes so fast. How we got here is what I’m interested in.
2018
2018 – Ted Talk: Christine Stabell-Benn: An unexpected vax vs unvax mortality study – WATCH
2017
May 2017 – WHO WHA – The Ministers of Health from 194 countries endorsed a new resolution on strengthening immunization to achieve the goals of the Global Vaccine Action Plan (GVAP) a roadmap to prevent millions of deaths through more equitable access to vaccines by 2020 – (via WHO Immunization coverage) ARCHIVE
- It recommends scaling up advocacy efforts to improve understanding of the value of vaccines and urgency of meeting the GVAP goals..The Secretariat will report back to the Health Assembly in 2018, 2020 and 2022 on the achievements against the GVAP goals and targets.”
- The main goal of the 2017 campaign with the theme #VaccinesWork is to raise awareness about the critical importance of full immunization throughout life, and its role in achieving the Sustainable Development Goals.
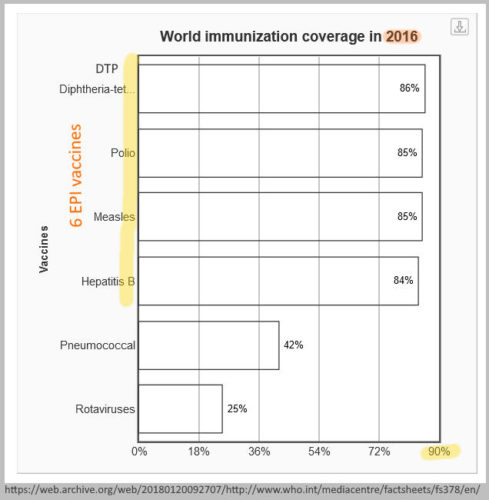
2015
October 12-16, 2015 – WHO Expert Committee on Biological Standardization: Collaborative study: Calibration of Replacement International Standard for Diphtheria Toxoid for use in Flocculation Test – PDF, ARCHIVE [what exactly is a vaccine???]
- “Diphtheria vaccines form an essential component of the primary immunization schedule of children and have been part of the WHO Expanded Programme on Immunization (together with tetanus and pertussis components) since its inception in 1974
- “Diphtheria is caused by exotoxin-producing strains of the bacterium Corynebacterium diphtheriae. Active immunization against diphtheria is based on the use of diphtheria toxoid (DTxd), a chemically detoxified preparation of diphtheria toxin, to induce protective antibody responses.”
- The bulk toxoid intermediates of diphtheria vaccines can also be used as carrier proteins in polysaccharide conjugate vaccines against invasive bacterial infections caused by N. meningitidis, H. influenzae and S. pneumonia“
- DTxd is produced by growing the toxin-producing C. diphtheriae in liquid media and converting the toxin to inactive toxoid by treatment with formaldehyde. Antigenic strength and purity of the bulk toxoid is evaluated by measurement of ‘limit of flocculation’ (Lf) units….” “Due to its simplicity, speed and economy, flocculation remains the primary method used by vaccine manufacturers to evaluate toxin and toxoid concentrations in Lf.”
2015 – SAGE: 2015 Assesment Report of the Global Vaccine Action Plan REPORT- PFD
- Decade of Vaccines half way point, Where are the unvaccinated people?
- “Coverage is hampered and only 30 to 40% of children are immunized against major childhood diseases. Inaccessible areas are home to more than half a million children still requiring catchup vaccination.”
2013
January 17-88, 2013 – WHO: Expert consultation on the use of placebos in vaccine trials – PDF
- “New and improved vaccines to prevent illness and death from infectious diseases are urgently needed, especially in low- or middle-income countries (LMICs)……” safety trials are done in randomized controlled trials but too often not with an inert placebo, but another vaccine product.
2012
May 2012 – WHO 65th World Health Assembly – SAGE Global Vaccine Action Plan (GVAP) 2011-2020 (resolution WHA65.17) framework was endorsed, to help realize the vision of the Decade of Vaccines launched by Bill Gates in 2010 – REF, READ, UNICEF – READ
- GVAP is “a framework to prevent millions of deaths from vaccine-preventable diseases by 2020″
- “an immunization vision and strategy for the world for the decade 2011–2020.” – PDF
- “GVAP aims to strengthen routine immunization, accelerate control of vaccine-preventable diseases with polio eradication as the first milestone, introduce new vaccines, and spur research and development for the next generation of vaccines and technologies.” – REF
2010
2010 – WHO Decade of Vaccines (2010-2020)
2010 – United Nations General Assembly Special Session (UNGASS) goals by 2010 – REF
- (i) Ensure full immunization of children under one year of age at 90% coverage nationally with at least 80% coverage in every district or equivalent administrative unit, (ii) Vitamin A Deficiency Elimination
- World Health Assembly (WHA) and UNGASS goals by 2005: (i) Polio Eradication, (ii) Measles Mortality Reduction, (iii) Maternal and Neonatal Tetanus Elimination (MNTE)
- Progress Towards Global Immunization Goals – 2012 – presentation slides – PDF
January 29, 2010 – BMGF: Bill and Melinda Gates Pledge $10 Billion in Call for Decade of Vaccines – ARCHIVE, GAVI celebrates 10 years – ARCHIVE, WHO – ARCHIVE, Bill Gates annual letter – ARCHIVE
- “US$10 billion over the next 10 years to help research, develop and deliver vaccines to the world’s poorest countries sets a new precedent in global heath.” – REF
- “Increased vaccination could save more than 8 million children by 2020; significant funding gaps remain, others must join effort” [vaccines “save” 2-3 million in 2020]
- “As Bill and Melinda emphasised, we need look no further than GAVI’s first 10 years for living proof that immunisation is one of the most cost-effective ways to save children’s lives” – REF
- “Despite these achievements…2.4 million children continue to die each year from vaccine-preventable diseases.”
- Important new vaccines for the two leading causes of global child deaths—severe diarrhea and pneumonia—are becoming available
- “As we build on that success, Bill & Melinda Gates’ call for a “decade of vaccines” represents the second time that the Gates Foundation is playing a transformational role in the delivery of life-saving vaccines to the world’s poorest countries” – REF
- “The Bill & Melinda Gates Foundation announcement comes on the tenth anniversary of the establishment of the Global Alliance for Vaccines and Immunization (GAVI). Dr Chan also congratulated the GAVI Alliance on their accomplishment of reaching 257 million additional children with new and underused vaccines.” – REF
January 2010 – (START) The WHO REFORM AGENDA traces back to “The future of financing for WHO – January 2010” – Timeline – “Landmark events of the WHO reform process from the initial consultation on the future of financing for WHO “-ARCHIVE, What is Reform – READ, 2014,
- Followed by 128th session of the Executive Board, January 2011
2006
2006 – WHO “Engaging for health” program covers the 10-year period from 2006 to 2015 – REAF
2005
2005 – WHO Techical Report Series: WHO EXPERT COMMITTEE ON BIOLOGICAL STANDARDIZATION – PDF
- “The production and control of conjugate vaccines is more complex than that of their unconjugated capsular polysaccharide counterparts. Polysaccharide vaccines consist of defined chemical substances that if prepared to the same specifications, can reasonably be expected to have comparable potencies” (pg 68) [so not biological material?]
- Includes definition for GMO, Immunogenicity, Poentcy, Primary vaccination etc but not “vaccine”
- “Many vaccines are produced using prokaryotic or eukaryotic microorganisms and subtle changes in these organisms may radically affect the vaccine product…”
November 9-11, 2005 – SAGE evolves into a body overarching global immunization, not just childhood immunization – REF
October 24-28, 2005 – WHO 56th meeting of Expert Committee on Biological Standardization: Guidelines for Assuring the Qualoity and Non Clinical Safety Evaluation of DNA vaccines – PDF, SOURCE
- “Vaccination involves priming the immune system of a host with an infectious agent or components of an infectious agent modified in a manner to assure that the vaccine does not cause any harm or disease to the host, but ensures that when the host is confronted with that infectious agent, its immune system can adequately control the invading organism before it causes any ill effect”
- A radically new approach to vaccination has been actively and vigorously developed since early 1990’s. This involves the direct introduction of plasmid DNA containing the gene encoding the antigen against which an immune response is sought into appropriate host tissues and the in situ production of the target antigen(s).
- Similarly, many aspects of the guidelines may be applicable to vaccines based on RNA…”
June 12-15, 2005 – Pan American Health Organisation: Sixth Annual Global Vaccine Research Forum in Salvador da Bahia, Brazil – “Cutting-edge Science on New Vaccines: Global Forum to Cover Latest Developments, Key Challenges”- READ, CREDIT
- “Major breakthroughs are occurring in the development of new vaccines. There are about 20 vaccines currently in use; an equivalent number of new or improved vaccines is anticipated within the next ten years.”
- “These vaccines will be a relatively inexpensive health intervention with a significant public health impact; however, they will cost more than vaccines in current use. WHO Member States last month adopted a resolution on a new Global Immunization Vision and Strategy, an important component of which is introducing new vaccines”
- Prospects for new vaccines against pandemic avian influenza, Acceleration of life-saving rotavirus, pneumococcal and meningitis vaccines…
- The Pan American Health and Education Foundation is a U.S. not-for-profit philanthropic organization that enjoys a unique partnership with the Pan American Health Organization based on our shared vision of health for all – REF, List of topics covered – HERE Formally the 1902 Pan American Sanitary Bureau – ARCHIVE
May 23, 2005 – WHO: 58th WHA – new International Health Regulations (IHR) are unanomously adopted by WHO Member states – ARCHIVE, REF, REF2, TIMELINE
- IHR are a “tool in the fight against the global spread of infectious disease” – IHR are “rules that countries must follow to identify disease outbreaks and stop them from spreading” i.e. member states are bound by international laws
- 2003 – WHO Department of Communicable Disease Surveillance and Response (CSR) is working towards global health security – epidemic alert and response (Epidemic and Pandemic Alert and Response) – ARCHIVE
2004
2004 – The Global Health Histories initiative was established by WHO in late 2004 – 2006, 2010
- History is very important because all major contemporary policy declarations have historical sections. Historical analyses are being used to justify contemporary agendas. The historian working in tandem with policy managers has and important role to play. Even when you look at recent recent pronouncements about the need to increase immunization coverage, you have references to the lessons learned from the global smallpox eradication program. You cannot learn lessons unless you understand the complex history of that program – says Dr Sanjoy Bhattacharya, Director, Centre of Global Health Histories – (download) – WATCH
- Presentations 2005-2007, 2005-2014,
- PUBLICATION: The Third ten years of the World Health Organization (1968-1977) – PDF, ARCHIVE
- This is first new publication within the global health initiative. It is a sequel to the previous two volumes published in 1958 and 1968, during the 20-year tenure of Dr Marcolino Candau as Director-General, first elected in 1953, then 3 more times.
- A new phrase entered the text of the second volume published 1968, namely ‘smallpox eradication’.
2003
2003 – WHO Dept V& B: Vaccines and Biologicals Catalogue 2003 -Lists documents produced and distributed by the World Health Organization’s Department of Vaccines and Biologicals (V&B) since its establishment in 1998 – PDF, SOURCE co signed Gro Harlem Brundtland
2003 – WHO – Laboratory Safety Manual – PDF, SOURCE
- “This specialized agency of the United Nations published the first edition of its Laboratory biosafety manual in 1983” Biosafety Lab Levels 1-4
- “Recombinant DNA technology involves combining genetic information from different sources thereby creating genetically modified organisms (GMOs) that may have never existed in nature before.”…concern resulted in the famous Asilomar conference held in 1975, where the first guidelines for recombinant DNA technology were proposed. (pg 52) – TIMELINE
2002
November 26-28, 2002 – WHO/IVR: Ethical considerations arising from vaccine trials conducted in paediatric populations with high disease burden in developing countries – PDF
- CLAIM: “Each year, millions of children in developing countries suffer from infectious diseases. Of these, more than 2.7 million children under five (WHO 2001 estimate) die from diseases that are potentially [no guarantee] preventable by vaccines.
- [This is an important claim which is parroted by WHO yet supply without citation]
- In light of these unacceptable [estimated!] rates of morbidity and mortality, the development or improvement of vaccines to meet the needs of children in developing countries continues to be one of the highest priorities, with the attendant requirement for an increasing number of trials to evaluate new vaccines.”
- A cautious approach is appropriate in the conduct of vaccine trials among children in any circumstances because of their particular vulnerability in view of their inability to give informed consent and their sometimes greater potential to adverse reaction to vaccines.”
2002 – WHO: State of the World’s Vaccines and Immunization – jointly released by WHO, UNICEF and the World Bank, charts the many issues surrounding vaccines and immunization in 2002 includes “arguing the power of vaccines and immunization as an effective public health intervention” – PFD [Setting the ground work for ramping up the vaccine agenda]
- Document highlights the immense strides made in global immunization since the mid-1990s.
- “Imagine a world without vaccines. Life-threatening diseases would present a daily risk… We would live in fear of deadly strains of diphtheria, tetanus and measles; polio would be a constant danger and in a matter of hours could paralyse a child, and smallpox would continue to scar and kill.” [Wow! someone should introduce the WHO to Dissolving Illusions!]
- “Immunization, as powerful and successful as it is, has yet to reach its enormous potential. One-quarter of the world’s children still have no protection from common preventable diseases. Nearly 3 million people (2 million of them children) die every year from those same killers. Children in developing countries are dying from other diseases, such as meningitis and pneumonia, while vaccines for these are widely used in the industrialized world.” …”The right to protection from preventable diseases is the right of every child and it is well within our collective capacity to realize that right.”
- [Another bold mortality claim with NO citation for how they came up with these numbers!]
- Smallpox was eradicated in 1979, polio is about to be eradicated [wrong] and about two-thirds of developing countries have succeeded in eliminating neonatal tetanus.
- But global commitment to immunization has not been sustained in all developing countries! By 2000, about 37 million children worldwide missed out on routine immunization during their first year of life…Today, the divide in access to vaccines and immunization continues to undermine the principle of equity on which national immunization programmes should be based.”…global immunization coverage of over 70% was sustained throughout the1990s…”
- “Despite the overall success of immunization programmes, almost 11 million children under five years of age die each year.” [Seriously they are just making up these numbers, it was 2.7 million in 2002]
- “In sub-Saharan Africa, for example, deaths among children under five almost doubled over the past four decades from 2.3–4.5 million a year”…Millions more children are growing up with no protection against some of the life threatening, disabling and vaccine-preventable diseases of childhood.” [EVERYTHING is vaccines!]
- …”lack of demand for a new vaccine at the outset can have a long-term impact on both the supply and price” – [and there you have it folks, need to keep creating demand!]
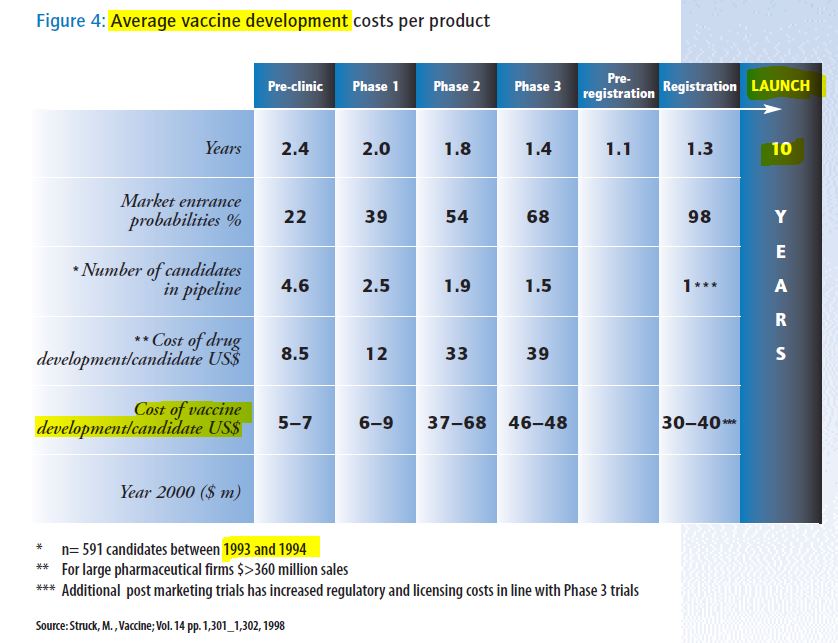
- “There are many reasons why the world community should invest in immunization and the reduction of infectious diseases. They include not only public health reasons but also humanitarian, economic and social reasons.”
- “While the market for vaccines in developing countries is potentially vast – including the 132 million children born each year – they currently account for only 18% of the global US$6 billion vaccine market.”
- “The global vaccines market size is projected to grow from $78.27 billion in 2023 to $122.27 billion by 2030, at a CAGR of 6.6% during the forecast period” – REF
2001
2001 – WHO Department of Vaccines and Biologicals – Biennial Report 2000-2001 –PDF
- GAVI was launched and changed the international vaccination stage
2001 – WHO “IMMUNIZATION systems are stagnating. Many countries that were dramatically successful in raising
their immunization coverage in the 1980s are now finding it difficult to lift coverage rates from 50% up to a target of at least 80%.” – REF
2000 – The Year of vaccine ramp up
December 2000 – WHO Technologies for vaccine delivery in the 21st century (Syringe, needles and storage) – PDF
- Sponsored by PATH etc
October 26-27, 2000 – Report of the second meeting of the Steering Committee on Immunization Safety (SCIS), Geneva – PDF (sponsored in part by Bill and Melinda Gates Children’s Vaccine Program (CVP))
- Up to 1/3rd of immunization injections today are not carried out in a way that guarantees safety.
September 2000 – UN Millennium Summit held in New York in – set the eight Millennium Development Goals (MDGs) – REF
- “The fourth goal, to reduce child mortality, has the specific target of reducing under-five mortality by two-thirds between 1990 and 2015. One of the specific indicators to measure progress towards the goal is the proportion of one-year-old children immunized against measles.” !
June 13, 2000 – WHO V&B: Networking for new vaccine evaluation – examine ways of helping national regulatory authorities (NRAs) to evaluate clinical data generated in support of vaccine licensing – led by TGA’s Dr John McEwen [i.e. Training the regulat -ors for the anticipated influx of vaccines] – PDF
June 11-12, 2000 – WHO Dept Vaccines and Biologicals (V&B): Report of the Strategic Advisory Group of Experts (SAGE) -their second meeting – PDF, SOURCE
- Accelerated vaccine introduction (AVI) of 1999, thanks to funding via the new entity GAVI – The aim of the project is to find and apply, by 2003, mechanisms for speeding up the introduction into developing countries of new and underused vaccines that could be of public health importance in these countries.”…”Major barriers include a lack of adequate financing, the absence of satisfactory data on vaccine efficacy and cost-effectiveness, and on the burden of vaccine target diseases, and the need for technical assistance on logistics, supply and quality control.”
- “SAGE believes vaccine and immunization safety to be one of the most important issues that the vaccine community faces. A major catastrophe that could have been prevented by timely, appropriate action could destroy the whole vaccine edifice. SAGE therefore strongly supports efforts to develop efficient, continuous monitoring systems and sound scientific evaluation systems, with a view to anticipating the occurrence of vaccine-related adverse events“
- “SAGE urges WHO to be in close touch with all the other players involved in the introduction of new vaccines, most specifically those that are devoting funds to vaccine introduction initiatives, such as the Bill & Melinda Gates Children’s Vaccine Program [Private] and the International Vaccine Institute, and also the vaccine industry, which is strongly promoting the development of new vaccines.”
- In light of GAVI’s introduction in 1999 – “SAGE would, as a result, have to assume a new role in a new universe – a universe in which for the first time the vaccine and immunization scene would benefit from significant financial resources, thanks to the influx of funding that attended the birth of GAVI.”
- “There has also been a change in the role of SAGE, as reflected in its designation as a “strategic” rather than purely “scientific” group of experts. This change denotes a broader definition of the scope of SAGE, which no longer focuses only or primarily on vaccine research and development but now encompasses the whole spectrum of V&B activities”
June 7-9, 2000 – WHO Proceedings of the first Global Vaccine Research Forum, Montreux – PDF
- Creating incentives for industry to invest in developing market vaccines: role of public sector in forging partnerships with industry
February 2000 – Bulletin of the World Health Organization, 2000; 78(2): 153-231 (Reprint); Immunization safety: a global priority – PDF
- In 1999 the Immunization Safety Priority Project (ISPP) began where by 2003 the WHO will establish a comprehensive system to ensure safety
- “When WHO began its immunization programmes in 1974, less than 5% of the world’s children were immunized. Twenty-five years later, the figure stands at about 75%….
- “Adverse events following vaccination (AEFI) range from the common, innocuous redness and soreness at point of injection, to rare, serious conditions such as the potential risk of a severe allergic reaction in 1:100,000 to 1:1,000,000 doses of measles vaccine.” [no citation was provided]
March 16-17, 2000 – Report of a meeting of international public sector vaccinology institutions – PDF, SOURCE
- Prioritizing vaccines for the developing world- “The priority vaccines of the WHO Intercluster Vaccine Research Initiative (IVR) include HIV, malaria, tuberculosis and specific developing market vaccines which have yet to be selected”
- Pilot production issues for parasitic vaccines – As there are currently no vaccines against parasites for human use [Could ivermectin actually be considered a oral ‘parasitic vaccine”, in that it is a biological, derived from an organism??]
- “The group stated the importance of combination vaccines for the immunization programmes in the developing world, their impact on the operational costs and associated safety issues.” with “Ongoing work to produce combination vaccines (DTP–HepB–Hib, measles/JE, MMR/varicella)”!!!
January 2000 – GAVI: A new multi-million dollar Global Fund for Children’s Vaccines (the Fund) was launched by the GAVI partners – Press Release – ARCHIVE
- “Enabled by a generous gift from the Bill and Melinda Gates Foundation ($150 million per year for five years, This is the largest gift in the Foundation’s history [It’s one month old!]), the Global Fund for Children’s Vaccines has been created by GAVI to help fill the gaps that currently prevent the immunization of each and every child against major diseases.” –REF
January 31, 2000 – The Global Alliance for Vaccines and Immunisation (GAVI) is launched – TIMELINE
- Sponsored by BMGF and launched a the WEF.
- “Since 2000, the GAVI partners have been targeting assistance to the poorest countries through the Vaccine Fund to help them boost coverage with existing vaccines, improve immunization systems (including injection safety) and introduce under-used vaccines, including hepatitis B, Hib and yellow fever” (2003)- REF
- “The culture of disease prevention gained momentum in the biennium, particularly through” the impressive debut of the Global Alliance for Vaccines and Immunization (GAVI)” – REF
- “The advent in 2000 of the Global Alliance for Vaccines and Immunization (GAVI) and of the Vaccine Fund have brought immunization back to the top of the international public health agenda.“- REF
1999
November 1999 – WHO Dept V&B: Study of donor inputs to vaccine production – PDF
- In 1991 studies were begun of the characteristics of local vaccine production (produced within a country for use in that country, generally in a public sector facility) in developing countries under the auspices of the Children’s Vaccine Initiative.
- WHO is now poised to play an enhanced role in ensuring access to new vaccine and immunization-related technologies, through
- (1) strengthening national regulatory authorities;
- (2) promoting local production viability; and
- (3) facilitating access to patent-protected technologies.
November 15-16, 1999 – WHO Dept V& B: Report of a meeting on health sector reform and priority health interventions: the case of immunization services – PDF
- “A substantial number of countries have embarked upon, or are in the process of planning fundamental changes in their health systems – health sector reform – to respond more adequately to the needs of local populations as well as to other challenges from the socioeconomic and political environment” [Really?]
- “The meeting was organized to take stock of the interrelationship between health sector reform and immunization services” which are said to be a means of “strengthening health systems” and “Immunization services performance is an excellent indicator of the performance of the overall health system”!
November 4-5, 1999 – Assessing the global needs for vaccine research and development. Results of a Joint GAVI/WHO meeting, Geneva w/ co-chairs GAVI, Aventis-Pasteur and GmithKlein Beecham – PDF, SOURCE
- “Recognising that “Accelerating the research and development efforts for vacciens and related products specifically needed by developing countries, particularly vaccines against HIV/AIDS, malaria and tuberculosis” is one of the fundamental objectives of the Global Alliance for Vaccines and Immunization, the purpose of this “pre-Task Force” on R&D”
- Meeting co-sponsored by the Intercluster Vaccine Research Initiative of the WHO
November 1-3, 1999 – Report of the Strategic Advisory Group of Experts (SAGE) with in WHO Dept Vaccines and Biologicals (Published March 2000) – PDF, SOURCE, GPV document ARCHIVE, ARCHIVE
- Report from the FIRST meeting of the SAGE (with”S” for “Strategic”, no longer “Scientific”) established by Dr Gro Harlem Brundtland, the Director-General of the World Health Organization in 1999 – TIMELINE
- “Report was funded by unspecified donors!”The Department of Vaccines and Biologicals thanks the donors whose unspecified financial support has made the production of this document possible” [Hmmm!]
August 1999 – WHO | Access to Technologies Team of the Department of Vaccines and Other Biologicals: Regulation of vaccines: building on existing drug regulatory authorities – PDF
- Although vaccines are generally included in the legal definition of pharmaceutical products, and thus would fall under the jurisdiction of drug regulatory authorities (DRAs), there are extra considerations that apply to their regulation and control.” …
- “Vaccines provide one of the most cost-effective of all public health interventions and are among the safest medicinal products.”
- “Vaccines differ from therapeutic medicines first because of the biological, and thus inherently variable nature of the products themselves, the raw materials used in their production, and the biological methods used to test them. Thus special expertise and procedures are needed for their manufacture, control, and regulation.” They are “usually administered to very large numbers of healthy people, mostly infants, in national immunization programmes; thus safety and quality are paramount.”
- New vaccines are being developed at a rapid pace and these vaccines will represent new and complex challenges for regulatory authorities as well as for vaccine manufacturers – i.e. conjugate vaccines and new DNA vaccines
- What are the essential features of a regulatory system for vaccines?
- Because of the special biological nature of vaccines, all 6 vaccine regulatory steps need to be followed, which includes the distribution and storage be well supervised, down to the end user.
- Staff expertise required for “areas of assessing the documentation for marketing authorization, Good Manufacturing Practice (GMP) inspections, and authorization and evaluation of clinical trials on vaccines.
- …due care should be exercised to ensure that the characteristics of the product which will be proposed for licensing are the same as those of the product which will be tested in humans” [They failed this step for COVID-19 Pfizer mRNA from Process 1 to Process 2]
- This means that associations supporting causality will be difficult to establish or rule out.
June 1999 – WHO: Options for a Global Fund for New Vaccines – PDF
- “Currently, a Global Fund for New Vaccines is being put forward as one possible part of a system for expanding and improving vaccination…” Exploring the five parameters of: equity, impact, feasibility, sustainability and scope. “Investment of fund capital is expected to provide returns of approximately 8% per year”.
- GAVI/BMGF to the rescue with Jan 2000 with the launch of the Global Fund for Children’s Vaccines, – ARCHIVE
1999 – WHO – Strategic Advisory Group of Experts (SAGE) on Immunization was established by the Director-General of the World Health Organization in 1999 to provide guidance on the work of WHO – ARCHIVE, ARCHIVE2 [
- The Terms or reference of the Group were revised during 2005 in view of the development of the Global Immunization Vision and Strategy (GIVS).
- Note the word “Strategic AGE” versus “Scientific AGE, yet both use acronym SAGE
- SAGE is the principal advisory group to the WHO for vaccines and immunization, for all vaccine-preventable diseases. It provides guidance on the work of the Department of Immunization, Vaccines and Biologicals (IVB). SAGE meets annually – REF
- SAGE is charged with advising WHO on overall global policies and strategies, ranging from vaccines and technology, research and development, to delivery of immunization and its linkages with other health interventions.
- SAGE has multiple Working Groups – EXAMPLE
- SAGE membership can include representatives of the Bill and Melinda Gates Foundation (BMGF) a WHO major sponsor, such as in 2013 Dr Steve Landry, Deputy Director of Global Health Program, Bill & Melinda Gates Foundation – ARCHIVE
- SAGE compose and release “Vaccine Position Papers” to provide recommendations to member states on vaccination – ARCHIVE, ARCHIVE2, all ARCHIVES, 2007 first – ARCHIVED, 2015 – ARCHIVE, 2022 – ARCHIVE
- Example Chickenpox vaccine 1998 – PDF
- “Varicella (chickenpox) is an acute, highly contagious viral disease with worldwide distribution. While mostly a mild disorder in childhood, varicella tends to be more severe in adults. It may be fatal, especially in neonates and in immunocompromised persons.”
- Example Chickenpox vaccine 1998 – PDF
- WHO recommendations for routine immunization – ARCHIVE, 2016 – ARCHIVE
January 21- 22, 1999 – WHO: | Department of Vaccines and Other Biologicals: Review of existing documents on planning, performance and assessment of clinical studies on vaccines – PDF
- Vaccines are a “special group of pharmaceuticals has distinct features that should be taken into consideration in planning, performance and assessment of clinical studies”
- “Many vaccines are intended for use in, and clinical studies in children necessitate especially careful planning and a thorough assessment of the risk–benefit ratio”
- The “most important internationally adopted documents covering the ethical aspects of biomedical research on human subjects”
- Control groups or “comparator may be either active or a placebo”
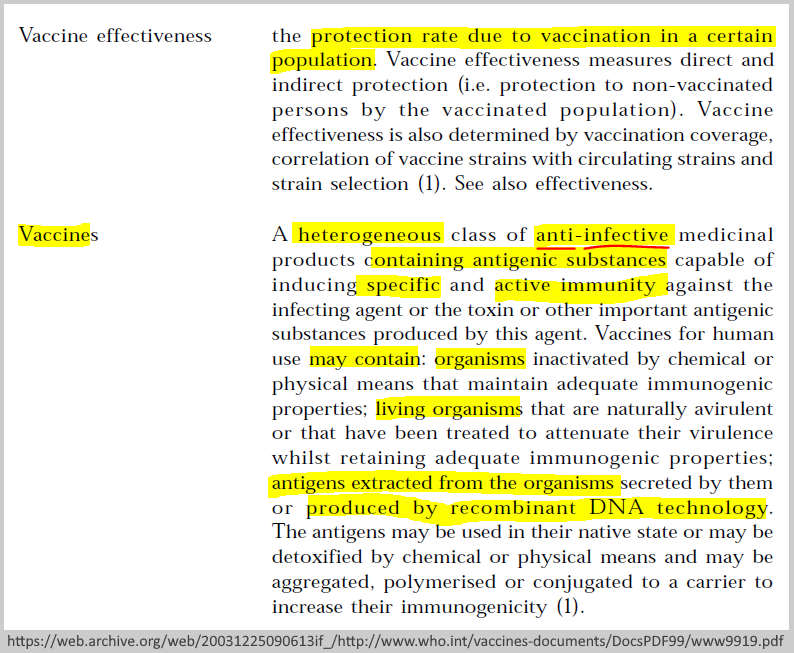

In 1999 – Bill Gates began to reposition his brand from computer geek monopolist to public health and education “philanthropist” by forming the Bill & Melinda Gates Foundation (BMGF) which officially launched in January 2000 –TIMELINE
1998
June 9-11,1998 – Report of the meeting of the Scientific Advisory Group of Experts (SAGE) – a joint Children’s Vaccine Initiative (CVI) and Global Program for Vaccines and Immunization (GPV) report – PDF
- “…thanks the donors whose unspecified financial support in 1997 has made the production of this document possible” [How can this group of experts be trusted as “independent” if funding sources are unknown with possible conflicts of interest?]
- This report is the result of “The third formal meeting of the Scientific Advisory Group of Experts (SAGE) for the Children’s Vaccine Initiative (CVI) and the Global Programme for Vaccines and Immunization (GPV) was held in Geneva on 9-11 June 1998″
- Terms of Reference of the Joint CVI and GPV Scientific Advisory Group of Experts (SAGE) – Page 72
- Note this Scientific Advisory Group of Experts “SAGE” is the prelude to WHO Strategic Advisory Group of Experts (SAGE) on Immunization, both with the same acronym! – TIMELINE
January 30, 1998 – WHO Department of Vaccines and Biologicals went online, formally known as the Global Programme on Vaccines and Immunization – ARCHIVE
1997
November 1997 – WHO: The Children’s Vaccine Initiative (CVI) Strategic Plan: managing opportunity and change : a vision of vaccination for the 21st century – READ, PDF The first strategic plan was 1992-93
- Published jointly by CVI, UNICEF, UNDP, World Bank, WHO and Rockefeller Foundation
- “global eradication of polio is now within immediate reach” – [they got that wrong again, but is good for marketing]
- CREDIT: Manufactured Consent (not infomed consent) by Suzanne Humphries, it started with this vision document – WATCH
1996
March 1996 – WHO: Global Programme for Vaccines and Immunization: Expanded Programme on Immunization (EPI) – PDF
- The first edition of the EPI document “Immunization Policy” was published 1986 which began by targeting “vaccine coverage”
- By 1996 “the EPI has changed its focus to the control or elimination of major childhood diseases, and new vaccines have become available, while yet others are being developed.”
- “The EPI recommends that all countries immunize against poliomyelitis, diphtheria, pertussis, tetanus and measles, and that countries with a high incidence of tuberculosis (TB) infection should immunize against TB.”
- Table 3.Vaccine efficacy and vaccine-induced immunity
- Basic immunization schedules and strategies
- Figure 1: Expected duration of immunity after different immunization schedules
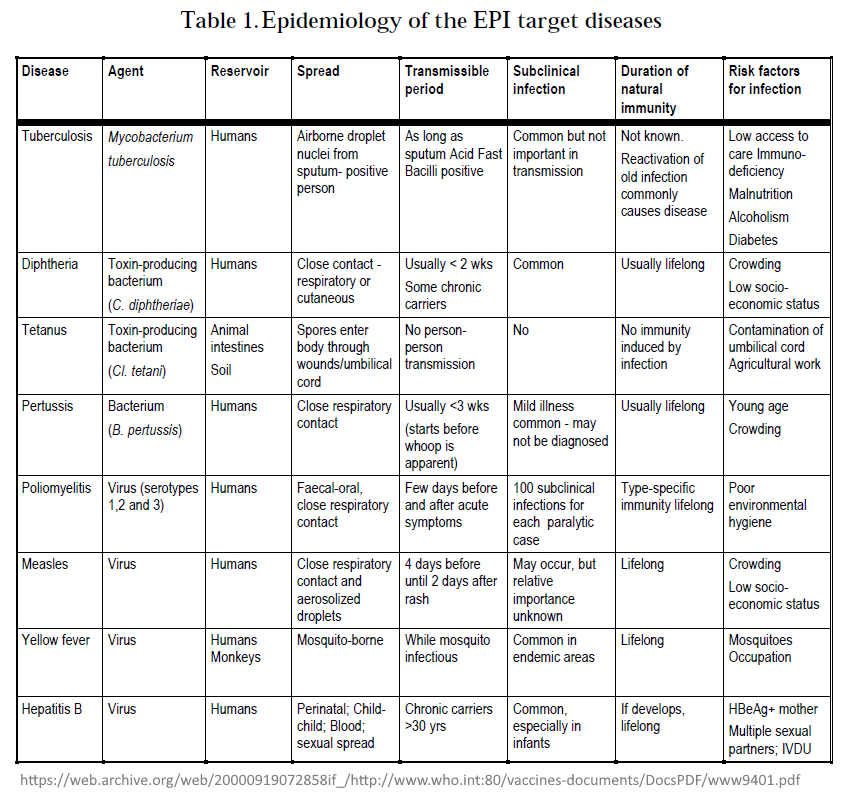
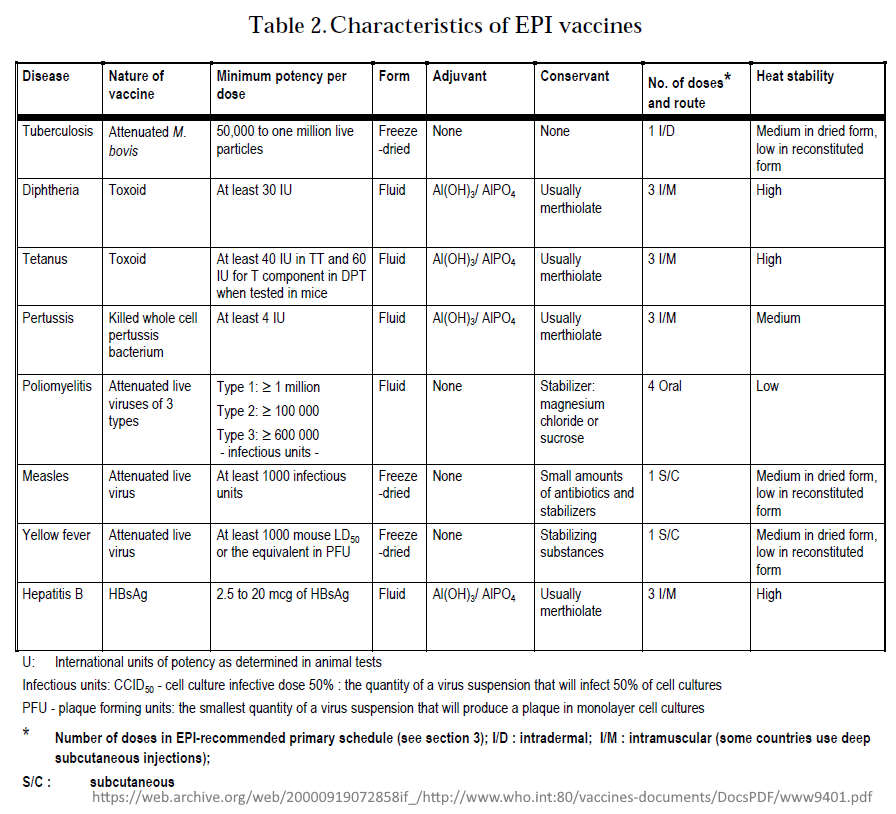
- Also found in side the document:
- Table 3.Vaccine efficacy and vaccine-induced immunity
- Basic immunization schedules and strategies
- Figure 1: Expected duration of immunity after different immunization schedules
- Until recently, the EPI has not addressed the issue of booster doses of EPI vaccines (EPI 1993e). The first priority has been to ensure that infants are completely immunized against target diseases at the youngest age possible – only consider boosters when coverage >80%!
- Table 9.Percentage of countries using different immunization schedules for DPT vaccine
- Reactions following immunization – “modern vaccines are safe”, blame most reactions on needles not being sterilized properly!
1995
June 12-14 – 1995 – WHO Vaccine R&D (VRD) Global Programme for Vaccines and Immunization (GVP) – SAGE: 1995 Progress Report & Plan of Activities for 1996 (WHO/VDR/GEN/95.03) – PDF
1993
1993 – WHO Immunological Basis for Immunization – Module series developed by WHO/EIP to answer questions from the field – Initially started with 8 modules- REF
1992
1992 – WHO | 45th WHA – Global Programme for Vaccines and Immunization – GPV Policy Statement on vaccine quality – resolved that all vaccines used within national immunization programmes meet WHO requirements (WHA45.17), thus reinforcing these guidelines as a credible goal for all countries – PDF
1990
September 29-30, 1990 – United Nations World Summit for Children – largest gathering of world leaders ever- READ, WIKI
- The Declaration on the Survival, Protection, and Development of Children was endorsed
- First Call for Children: World Declaration and Plan of Action from the World Summit for Children – Conventions of the Rights of the Child – a person under 18 years – PDF
- Childrens Vaccine Initiative (CVI) was launched
May 1990 – World Health Assembly set measles vaccine goal – REF
- “Reduction by 95% in measles deaths and reduction by 90 per cent of measles cases compared to pre-immunization levels by 1995, as a major step towards the global eradication of measles in the longer run”.
1989
1998 – UN Foundation : UN Human Rights Convention, The Convention on the Rights of the Child, was adopted unanimously by the United Nations General Assembly in 1989 – REF,
- “The Convention breaks new ground in international law by legitimizing the needs of children, and outlines the minimum requirements for their legal and physical well-being. In particular the Convention defines the rights of all children to healthy and productive lives.” [vaccines = “health” to UN/WHO]
- The United Nations Children’s Fund (UNICEF) is the only UN agency exclusively dedicated to children’s issues, including children’s health. It “helped to marshal international policy agreements on behalf of children. Most notable is the Convention on the Rights of the Child” One of UNICEF’s “primary missions consists of preventing and treating childhood illness and death.”
May 1989 – WHO: 42nd World Health Assembly set the agenda for the Expanded Programme on Immunization (EPI) in the 1990s. – REF
- Challenges included the reduction of measles incidence and elimination of neonatal tetanus by 1995, global eradication of poliomyelitis by the year 2000, and the achievement of 90% immunization coverage for all vaccines by the year 2000.
1986
1986 – WHO: Global Programme for Vaccines and Immunization: Expanded Programme on Immunization (EPI) document “Immunization Policy” – which was used as “a basis for immunization programmes throughout the world.” – REF – Setting targets for “vaccine coverage” by 1990.
- In 1986 “… many programmes were in the early stages of development and global goals referred to the achievement of coverage targets” By 1996 the EPI focused on control of elimination of major childhood diseases
1983
1983 – A New Pandemic emerges HIV/AIDS- Much of the world’s optimism about the state of health was shattered by a new, mysterious pandemic, HIV/AIDS. In Australia, the virus was first identified in a patient in Sydney in 1983 – REF, REF
1983 – The First edition of Laboratory Biosafety Manual was published in 1983 – REF, SOURCE
- “The manual encourages countries to prepare specific codes of practice for the safe handling of pathogenic microorganisms in laboratories within their geographical borders, and provides expert guidance for developing such codes of practice.”
1981
1981 – WHO: World Health Day with the ‘Health for all by the year 2000‘ – REF
1981 – Vaccines available in 1981 – REF
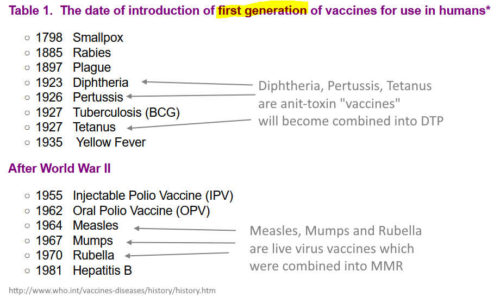
1980
1980 – Because of 1974 EPI extensive training program, by 1980 “almost every country in the world adopted the principle of a national immunization programme – on the back of 6 vaccines – REF
1980 – WHO declared smallpox eradicated – now then need a new enemy to targed
- Somewhere in our history “vaccine” and “vaccination” has come to mean something very different to it’s original definition
- Vaccine = cow (vacca) pox inoculation to ward of smallpox
- Vaccination = Coined by Edward Jenner for the act of “vaccine inoculation to ward of smallpox”
1979
December 9, 1979 – Global Commission for Certification of Smallpox Eradication – sign parchment at Geneva – Archives of the Smallpox Eradication Programme –ARCHIVE, WHO Smallpox – 2014
- “The last known natural case was in Somalia in 1977. Since then, the only known cases were caused by a laboratory accident in 1978 in Birmingham, England, which killed one person and caused a limited outbreak. Smallpox was officially declared eradicated in 1979 ” FAQ – ARCHIVE
- “When smallpox was officially certified as eradicated, in December 1979 an agreement was reached under which all remaining stocks of the virus would either be destroyed or passed to one of two secure laboratories – one in the United States and one in the Russian Federation. That process was completed in the early 1980s and since then no other laboratory has officially had access to the virus which causes smallpox.” – REF
- In 1980 the 33rd World Health Assembly endorsed the conclusions of the Global Commission for Certification of Smallpox Eradication that smallpox had been eradicated worldwide and that the return of the virus was unlikely – REF
- Smallpox is now a disease covered under WHO Global Alert and Resonse (GAR) – REF, originally Communicable Disease Surveillance and Response (CSR) – ARCHIVE
1978
September 12, 1978 – International Conference on Primary Health Care, in Alma-Ata, Kazakhstan sets the historic goal of “Health for All” – REF, TIMELINE
1977
1977 – The first Essential Medicines List appeared in 1977, two years after the World Health Assembly introduced the concepts of “essential drugs” and “national drug policy” – REF
1974
May 23, 1974 – WHO launch of the Expanded Programme on Immunization (EPI) – all the world’s children should receive 6 vaccines – TIMELINE, REF, REF2, 50 year anniversary in 2024 – READ
- “Following the impressive success of the smallpox eradication programme, the World Health Organization looked for other activities that could build on what had already been achieved. In 1974 the Expanded Programme on Immunization was created. “Expanded” because most programmes until then had only used smallpox, BCG and diphtheria, tetanus and pertussis (DTP) vaccines. EPI would include two new diseases. The six diseases chosen were tuberculosis, diphtheria, neonatal tetanus, whooping cough, poliomyelitis and measles.” – WHO History of Vaccines – REF
- “Selection was made on the basis of a high burden of disease and the availability of a well-tried vaccines at an affordable price. “Expanded” also meant increased coverage – incredibly, less than 5% of children in developing countries were being reached at that time by immunization services.” UNICEF and Rotary International partnered with EPI
- “Building on the momentum of the smallpox eradication effort, EPI was initiated with the goal of providing universal access to life-saving vaccines for children worldwide…” And so begins the mass, systematic “inoculation” program
- The programme developed training materials and disseminated them widely. By 1980 “almost every country in the world adopted the principle of a national immunization programme.” – REF
- It is claimed in 1974 when WHO started its immunization services less than 5% of the world’s children were immunized. 25 years later, the figure stands at about 75% – REF
- By 2024 the WHO recommends 13 vaccine antigens, including COVID-19 – REF
1969
July 25, 1969 – Twenty-second World Health Assembly adopts “a revised and consolidated version of the previous International Sanitary Regulations” renamed the International Health Regulations (1996) – WHA – ARCHIVE, IHR – ARCHIVE, READ, PDF
- Following special review of the International Sanitary Regulations by the Committee on International Quarantine adopts the International Health Regulations (WHA22.46) annexed to report – PDF
1968
1968 – Second edition publication of the history of WHO a was when “a new phrase entered the text of the second volume, ‘smallpox eradication’.” – PDF
- “This occurred because, in 1958, the Eleventh World Health Assembly unanimously adopted a resolution initiating a worldwide programme for eradication of the disease”
1967
1967 – Official start year of the WHO Smallpox Eradication Program – which was declared eradicate in 1980 – REF
1966
1966 – The 19th World Health Assembly requested the Director-General of WHO to initiate action to carry out a world-wide smallpox eradication programme- REF
1961
1961 – WHO” At the 13th World Health Assembly (1960)- WHO publish their first immunization schedule – including smallpox vaccination, DTP, BCG, and TAB – REF
- Smallpox Eradication discussed “emphasizing the urgency of of achieving world-wide eradication” from p23 – PDF
- Establishment of National Public Health Cadres (WHA13.36)

1958
May 28, to June 13 1958 – Eleventh World Health Assembly (WHA) – Eradication of Smallpox Resolution (WHA11.54) was unanimously adopted – WHA official minutes READ, PDF, ARCHIVE, CREDIT
- It was deemed an opportune time “to raise the problem of the world-wide eradication of smallpox in the near future”
- Smallpox control has been raised since the 3rd WHA as noted “Having regard to the decisions and pertinent practical measures adopted by WHO for smallpox control and the intensification of antismallpox programmes, in particular resolutions WHA3.18, (Executive Board) EB11.R58, WHA6.18, EB12.R13, EB13.R3, WHA7.5, WHA8.38 and WHA9.49″
- “investigation of the means of ensuring the world -wide eradication of smallpox, taking into account the fact that smallpox persists in certain areas despite repeated vaccination campaigns
1958 (Published) – History: The First Ten Years of the World Health Organisation (1948-1957) – READ, SOURCE
- Part I – Evolution of International Public Health – CH 1,
- “International public health had its origin, just over a century ago, in the International Sanitary Confernce which opened in Paris on 23 July, 1851.”
- Part II – Establishment of the World Health Organisation
- Part III – Ten Years of Work
1956
May 8-25, 1956 – Ninth World Health Assembly – PDF, SOURCE International Certificate of Vaccination
- Resolution WHA9.49 – Under International Quarantine “Additional Regulations of 23 May 1956 amending the International Sanitary Regulations with respect to the Form of the International Certificate of Vaccination or Revaccination against Smallpox”

- “The intensified struggle against malaria would be impossible were it not for the mobilizing of WHO’s resources side by side with the resources of UNICEF -the transport, the sprayers and the DDT” [pg57] DDT is mentioned 43 times in the document.
- “Perhaps no two organizations in the United Nations are associated by the very nature of their activities as closely as WHO and UNICEF. You [WHO] give assistance in the form of technical knowledge and advice ; we [UNICEF] give it in the form of supplies, equipment and training”
1955
May 10-27, 1955 – Eighth World Health Assembly – PDF, SOURCE
- Resolution WHA8.38 Campaigns against Smallpox
- “URGES again that health administrations conduct, wherever necessary, campaigns against smallpox as an integral part of their public -health programmes”
- May 26, 1955 – WHA8.30 on Malaria Eradication [think polio incidence]
- The “ultimate goal of malaria -control programmes should be the eradication of the disease”
- It is recognised that mosquito species Anopheles sacharovi are resistant to DDT in Greece and in Panama, Anopheles albimanus exhibited strange behaviour “after some six years of exposure to DDT, began in one area to avoid treated surfaces…” Such behaviour characteristic, if widespread, would of course make DDT useless for malaria control.”
1954
May 4-21, 1954 – Seventh World Health Assembly – PDF, SOURCE
- Resolution WHA7.5 Campaigns against Smallpox
- “Considering that Article 2(g) of the Constitution provides that a function of the Organization shall be “to stimulate and advance work to eradicate epidemic, endemic and other diseases“
- …continue studies on the most effective methods of smallpox control” etc
- Also note: WHA7.50 – Relations with UNICEF – “…Recognizing that UNICEF, originally an emergency organization, has recently been put on an indefinite basis”
1953
May 5-22, 1953 – Sixth World Health Assembly – PDF, SOURCE, credit 11th WHA 1958
- Resolution WHA6.18 Study on Campaign against Smallpox following executive board resolution (EB11.R58)
1952
1952 – WHO: Global Yaws control programme a tropical bacterial disease – REF, SOURCE, Wikipedia –READ
- “One of the first diseases to claim WHO’s attention was yaws, a crippling and disfiguring disease that afflicted some 50 million people in 1950. The global Yaws control programme, fully operational between 1952-1964, used long-acting penicillin to treat yaws with one single injection” (to which they have no vaccine “yet”!)
1951
May 25, 1951 – Fourth World Health Assembly – WHO Member States adopted the International Sanitary Regulations (ISR) (resolution WHA4.76), following the WHO Regulations No. 1 of 1948 which would later evolve into the International Classification of Diseases (ICD) – READ, REF
- WHA4.75 adn 4.76 – Adoption of the International Sanitary Regulations (WHO Regulations No. 2) –
- IHR were originally intended to help monitor and control six serious quarantinable infectious diseases: cholera, plague, yellow fever, smallpox, relapsing fever and typhus which were reported in the WHO Weekly Epidemiological Record (WER). Today, only cholera, plague and yellow fever are notifiable diseases.” [4]
1950
May 8-27, 1950 – Third World Health Assembly – The first WHO WHA Resolution made for smallpox (WHA3.18) – PDF, SOURCE, credit 11th WHA 1958
- “REQUESTS the Expert Committee on Biological Standardization to consider the question of the establishment of a centre for the testing and standardization of smallpox vaccines, with particular reference to dried vaccine and RECOMMENDS that greater weight should be given to smallpox in the regular programme for 1952” – Pg469
- References to DDT for controlling mosquitoes – vectors for malaria and yellow fever. Consider the statements
- “DDT, penicillin and other drugs have greatly extended the possibility of preventive care and remedial measures being made available to the people.”
- Yellow Fever: “DDT and other larvicides and insecticides-an inexpensive and effective way of destroying not only the Aedes aegypti [mosquito] but other vectors of disease”
- Re Typhus and other Rickettsioses: “WHO had sent technical experts, vaccine and DDT.”
- Annex 6 – Availability of DDT insecticides for combatting malaria in Agricultural areas from July 14, 1949 – “Recommends that Member Governments facilitate as much as possible the freer flow into the countries where they are needed of insecticides…”
- This is important information when you consider the trend in DDT use and polio incidence around this time – DDT is a highly persistent pesticide, and clearly recommended by the WHO. DDT poisoning looks a lot like polio! – READ
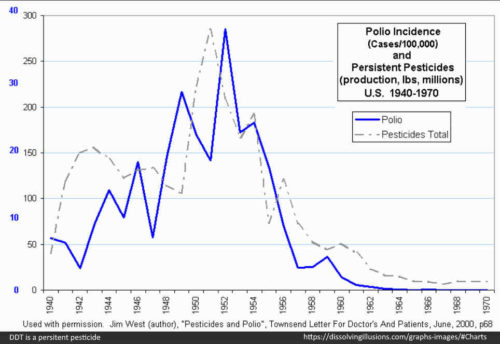
- “Between 1936, when his Government’s Malaria Division had been founded, and 1945, when the use of DDT had begun, campaigns against malaria had been carried out according to the methods then generally accepted. At the end of 1945, DDT spraying had begun in Venezuela” – [pg244 1956]

1948
1948 – In 1948 the WHO took over the responsibility for the International Classification of Disease (ICD), which dates back to the 1850s and was first known as the International List of Causes of Death. – REF, SOURCE
1947
1947 – WHO – Expert Committee on Biological Standardization meets on an annual basis since 1947 and is responsible for the establishment of the WHO International Biological Reference Preparations and for the adoption of the WHO Recommendations and Guidelines. – vaccines are “biologicals” – 2005 ARCHIVE
- Technical report series 2nd (1949) to 54th (2003) report – REPORTS
- 2002 Thiomersal in vaccines – WHO consider the the real benefits of these vaccines vastly outweigh the theoretical risk of thiomersal – so jab away until we get a replacement –READ
- 1994 DNA began discussion – READ
1946
1946 – Documentary: DDT Versus Malaria: A Successful Experiment in Malaria Control (released 1947), by the Kenya Medical Department – EXCERPT
June 8, 1946 – JAMA Vol 131 – Accidental ingestion of DDT, with a note on its metabolism in man- by M. I. Smith MD – pg 519 – READ
- First authentic report of the toxicity of DDT on man – CREDIT
- JAMA record notes urine analysis of man – where a 5% solution of DDT insecticide spilled onto chewing tobacco in man’s pocket ….he vomited… “became nauseated and apprehensive and experienced a sensation of tightness, [transient] stiffness and pain of the jaws and soreness of the throat.” – CREDIT
- [why am I documenting these data points? – because DDT’s “transient stiffness” aka paralysis <48hrs was diagnosed as “polio” up until 1955 (after release of Salk vaccine), before they altered the diagnostic definition of Polio]
1946 – DDT Killer of Killers – “In the summer of 1946, a poliomyelitis epidemic swept the country — the worst epidemic since 1916” – REF
- “In 1945, almost 33 million pounds of DDT were produced in the United States…Production was stepped up even higher in 1946…” – REF
July 22, 1946 – The Constitution of the World Health Organization (WHO)was signed in New York on July 22, 1946 on behalf of sixty-one States”, and came into force 2 years later on April 7, 1948 – TIMELINE
- The United Nations began operation October 24, 1945, under which the WHO was formed – TIMELINE
1945
1945 – DDT use began (important in reference to polio) – [pg244 WHA 1956]
April 21, 1945 – British Medical Journal – first case study reported of DDT poisoning via skin – developing paralysis like symptoms – READ, CREDIT

1945
1945 (onward) – Archived documents from Dr. Szeming Sze who was instrumental in the creation of the World Health Organization (WHO) through the United Nations delegation (many PDFs) – ARCHIVE, WHO constitution – TIMELINE
1934
1934 – DDT is rediscovered “in the scientific laboratories of JLR. Geigy, A. G., of Basle, Switzerland”, name dichloro-diphenyl-trichloroethan (DDT)
- [ BOOK: DDT: Killer of Killers by O.T. Zimmerman – READ]
- Then in 1939 the Colorado potato beetle came to Switzerland and an experiment with “a 1 % DDT dust” was successfully used. It was then used on heads to kill lice- REF
- In 1943, DDT was in commercial production at the Cincinnati Chemical Works, a subsidiary of Geigy Company, Inc., and early in 1944, Du Pont, Merck, and Hercules Powder Co. also went into production.”
1926
1926 – Australia hosted the League of Nations’ first international conference on health – REF
- The delegates discussed early warning systems for epidemics and quarantine issues, as well as various medical problems… then the Great Depression hit followed by World War II, afterwhich the World Health Organisation is formed in 1946






