COVID-19 Pandemic
2021
Timeline of significant global, Australian data points continuing into the second year of the COVID-19 Pandemic.
The first year of mass global rollout of brand new, experimental genetic technology products, classified as vaccines and the vaccine mandate rollouts!

Navigate the timeline pages:
1800s | 1900-1945 | 1946-1979 | 1980-1999 | 2000-2015 | 2016-2018 | 2019 | 2020 | You Are Here | 2022 | 2023 | 2024 | 2025
Quick links to months of 2021:
Jan | Feb | Mar | Apr | May | Jun | Jul | Aug | Sept | Oct | Nov | Dec2021 still requires a lot more data points, which in time will be added.

Vaccine Adverse Event reports exceeds CDC’s expected daily maximum
By January 1, 2021 daily Vaccine Adverse Event Reporting System (VAERS) reports received by CDC’s contractor General Dynamics Information Technology (GDIT) exceeded the expected worse case scenario of 1,000/day, this actually happened within 6 days of US roll out. [1]
On August 27, 2020 the CDC awarded GDIT a $35.4 million contract to manage the incoming VAERS reports. The FOIA’d “contract states that they were expecting up to 1,000 VAERS reports to be filed per day, with up to 40% of the reports being serious in nature”, up from the historical 5%. Reports exceeded 4,500/day on January 11, 2021.
Stories of vaccine injury were flooding social media in early January…but these were censored by Facebook and Twitter!
A month later on February 12, 2021 The New York Times reported that 34 million Americans had received a COVID-19 vaccine, but that “numerous federal health officials” had told them the FDA safety monitoring system “won’t be capable of analyzing safety data for weeks or months”! The Times also stated that “few serious problems have been reported through” VAERS and”no deaths have conclusively been linked to the vaccines.”
In February GDIT was asking for a 7 digit ID code to cope with the huge influx, as well as additional symptom codes, they reported processing a “record high” of over 40,000 VAERS reports in February, but have a backlog of 115,000 reports, and by March GDIT renegotiated their contract to accommodate a “minimum” of 25, 000 reports/week which approximates to 3570/day! In March they processed 60,200 reports, but were still “unable to keep up with the increased surge in reports”. They empolyed “over 90” backlog staffing positions – which continued every month for 2021. [2]
Mean while Fauci and Biden et al were claiming safe and effective!
Public not informed of failed COVID-19 vaccine efficacy
By January 4, 2021, apart from an editorial by Dr Peter Doshi in the British Medial Journal, the top 5 other medical journals “abjectly failed in their responsibility to inform the public” of the critical failure of the new injectable products, classified as COVID-19 vaccines, that with the data available had an efficacy of likely only between 19 to 29%, which failed to meet the minimum 50% efficacy standard for approval. [1]
New Beta variant escapes vaccine “immune protection”, starts talk of booster shots
As early as January 4, 2021, within weeks of the COVID-19 vaccine roll-out in the UK, their scientists expressed concerns that the new South African virus variant (501.V2) may render the new vaccines totally ineffective due to the spike protein mutations and “escape from immune protection”. [2]
The South African variant (B.1.351), later referred to as the Beta variant of concern (VOC), is thought to escape vaccine induced immunity. The virus mutations in the spike protein region, the target site for all COVID-19 vaccines, is a concern and has already prompted talk about developing booster shots. [1, 3]
“Developing” implies the manufactures are looking to “tweak” the spike protein mRNA code, which technically would require more clinical trials. They didn’t do this initially, boosters initially meant more jabs of the same Wuhan variant.
Israel is used as a pseudo “clinical trial” for Pfizer’s mRNA COVID-19 vaccine
On January 7, 2021 Israel’s (politically motivated) Prime Minister Benjamin Netanyahu announced his Operation Getting Back to Life deal with Pfizer Chairman and CEO Albert Bourla to exclusively use Pfizer’s COVID-19 vaccine product to vaccinate “all citizens of Israel over the age of 16 by the end of March and perhaps even earlier…” Making Israel “a global model state for the rapid vaccination of an entire country.” “We can do this because our health system is among the most advanced in the world”, being highly-digitalised. [1]
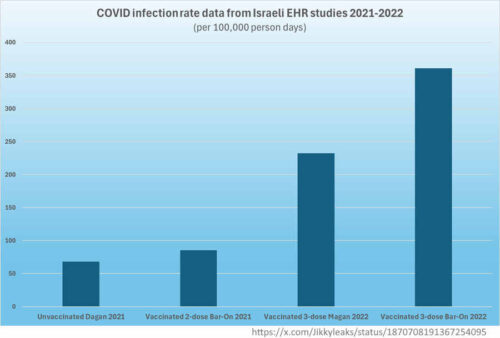
In exchange for Pfizer bringing forward vaccine deliveries, “Israel will share with Pfizer and with the entire world the statistical data that will help develop strategies for defeating the coronavirus.” – in effect making the country a mass test case to see how vaccines might halt the pandemic. [2, 7]
A new study was published in NEJM every time a booster dose was promoted for a new variant to “prove efficacy“, where each study used the less vaccinated as a control group for the “extra dose” group! [3, 4, 5, 6]
In the framework of one of the agreements “The Real World Epidemiological Evidence Collaboration Agreement” dated January 6, 2021 [8], the research outcome measures were defined by Pfizer. “None of the outcome measures that were explicitly agreed upon in advance were safety outcomes, such as overall mortality, hospitalisations from any cause or the known side effects of vaccines, whatever they may be.” [7]
“In effect, the contract between Pfizer and Israel’s Ministry of Health enabled the pharmaceutical giant to conduct a real-world experimental study of its Covid-19 mRNA vaccine efficacy using the Israeli population as test subjects”, and use it to promote alleged “effectiveness”. [7]
Pfizer had “the power under the agreement to omit any reference to its contribution to the research, so its involvement in setting research goals, methods or even in writing the research results is not mentioned at all. Thus, a study may be portrayed as independent of Pfizer, although it is not necessarily so.” [7]
US: Thawed vaccine shots going to waste
From as early January 8, 2021 [1] reports began to emerge across the US, that health facilities were throwing out unused and spoiled COVID-19 vaccines.
“If a clinic has a no-show for a vaccine appointment that extra dose cannot be saved for the following day. Doses expire quickly after they’re thawed.” [1, 2, 3]
On January 4, 2021 the first US pharmacies start to administer the vaccines include Walgreens, CVS, & Walmart.
US remove vaccine allocation for states
On January 12, 2021 The US government reversed course and is now releasing its entire cache of COVID-19 vaccines to states — including doses previously reserved for second shots, Health and Human Services (HHS) czar Alex Azar, reasoned this to be because they “now believe that our manufacturing is predictable”.
The two vaccines currently authorised, Pfizer & Moderna, require a second doses, 21-28 days following the first dose. “The Trump administration had been holding back millions of doses to ensure that those who received the first shot would get their second.”
States are now encouraged “to immediately open up immunizations to everyone age 65 and older.”
Sinovac vaccine 50.4% efficacy
Sinovac is a Beijing-based bio-pharmaceutical company developing the SARS-CoV-2 vaccine called CoronaVac, an inactivated virus particle vaccine.
On January 13, 2021 the Brazillian clinical trial results were announced and found the vaccine to have 50.4% efficacy, BARELY over the 50% needed for regulatory approval.
Norway: 23 Elderly die following vaccine
As of January 14, 2021, 23 reports of deaths following COVID-19 vaccination had been reported to the Norwegian adverse reaction register. The Norwegian Medicines Agency have linked 13 of these deaths to “common” vaccine side effects – these were elderly nursing home patients, the very risk group we are trying to help. [1, 2]
13 of these deaths are in nursing home patients and “the reports may indicate that common side effects from mRNA vaccines, such as fever and nausea, may have led to deaths in some frail patients,” says Sigurd Hortemo, chief medical officer at the Norwegian Medicines Agency (NMA).
The NMA noted that the vaccine clinical trials “on which the temporary approval of the vaccine is based included very few people over the age of 85. We therefore know little about how any side effects will affect the very elderly.”
A week earlier, on January 7, 2021, 2 nursing home residents died a few days after receiving their Pfizer vaccine. Then two days after this report the post-vaccination death count rises to 29, but the age group affected is lowered to 75 years.
The UK watchdog says vaccine reactions are “normal”, following “33 elderly people living in nursing homes [who] died shortly after being immunized.”
Stories of vaccine injury and death floods social media
By January 14, 2021, just a few weeks after vaccine roll-out in the UK, US & Israel, reports of serious injuries and death following COVID-19 vaccinations have been flooding social media.
Drive-through vaccine “super-stations” were set up across the world to vaccinate as many people as possible, healthcare workers first, to get to “herd immunity”. [1, 2, 3]
A collection of allergic reactions were thought to come from a particular batch of 33,000 Moderna vials distributed across the US, providers were asked to “delay” administering that batch.
US Fact Sheet on WIV – risky research and military ties
On January 15, 2021 the US the State Department released a fact sheet on the Activity at the Wuhan Institute of Virology (WIV) which disclosed that the United States was aware that the lab had been conducting risky research on coronaviruses since 2016 and was conducting Secret CCP military reasearch at the lab. A fact that the NIH, NIAID and their head the HHS were all aware of. [1, 2]
WHO Emergency Committee continue PHEIC
On January 15, 2021 the WHO Emergency Committee decided to continue the Public Health Emergency of International Concern (PHEIC) due to the “threat” of virus “variants”. The PHEIC is a necessary declaration to justify “emergency use” of vaccines.
At this point “the impact of vaccines in reducing transmission is yet unknown.”
The Fauci/COVID-19 Dossier released
Dr David Martin with his company M-CAM have since 1999 monitored patent activity in the arena of ‘health’ and compiled the comprehensive 205 page public document titled “The Fauci/COVID-19 Dossier” highlighting numerous breaches and violations of international law by the “experts” and agencies funded by taxpayers, and supplying all links to source material.
Martin states that the Dossier is
“one place to tell the story of corruption” and
“Crimes go unpunished if law enforcement don’t do their job.”
WHO: Medical Product Alert for PCR tests – Ct above 30 likely false positive
Just one hour after US Presidential Inauguration of Joe Biden, the WHO released a “Medical Product Alert” for PCR tests to “clarify” the previous Jan 13, 2021 notification, reiterating that false positive cases will occur if the PCR cycle threshold (Ct) is set too high. In short they now advise labs/physicians to be mindful of the need for clinical symptoms and to not use high amplification cycles (Ct 40 to 45), but to dial it back (Ct 30) and “manual adjustment” may be necessary. [1, 2]
On December 14, 2020 the WHO already warned about PCR cycle thresholds were too high, just as the vaccine roll out began!
No longer is PCR the “gold standard” for diagnosis, they refer to it now as “an aid for diagnosis”, meaning used in conjunction with other factors before declaring a diagnosis! Exactly what the alleged “skeptics” have been warning since November 2020 as the test is done on healthy, sympton-less people and a positive result renders that person statistically ill and is misleadingly considered “asymptomatic”. [1, 2]
Compare this to the WHO’s criteria for accepting a case of MERS in 2014, a positive antibody test was needed – proof of infection.
Throughout 2020 many global experts have been calling out the high false positive rates from the PCR test, which when used on masses of healthy people caused high numbers of “cases“, especially just before the November 3rd, 2020 US Presidential Election.
Each time PCR goes through a cycle, it doubles it reproduction, called amplification. The high number of cycles are known to return a high level of “false positives”. Any positive result was considered a “case” statistic and that person was “diagnosed”, often solely on the test, as infected, which is why the term “casedemic” emerged globally.
WHO Advice to healthcare professionals re COVID-19 vaccines
As per WHO Regulatory Update on COVID-19 they provide guidance to healthcare provides on how to answer COVID-19 vaccine related questions.
Their first claim is “there are still few effective anti-viral medicines for treatment of COVID-19 infection”, thereby justifying a vaccine. This is contrary to the experience of many frontline doctors.
TGA grants first COVID-19 vaccine Provisional Registration – Pfizer-BioNTech
On January 25, 2021 Australia’s TGA grants Provisional Registration for Pfizer Australia Pty Ltd‘s mRNA COVID-19 Vaccine for 16 years and older, the first vaccine for COVID-19 to receive “provisional approval” in Australia and the first new gene technology vaccine ever used. [1, 2, 3]
COMIRNATY (BNT162b2 [mRNA]) COVID-19 Vaccine has provisional approval for the indication below:
- Active immunisation to prevent coronavirus disease 2019 (COVID-19) caused by SARS-CoV-2
- The use of this vaccine should be in accordance with official recommendations.
- The decision has been made on the basis of short term efficacy and safety data. [clinical trial]
- Continued approval depends on the evidence of longer term efficacy and safety from ongoing clinical trials and post-market assessment.
This product, like all provisionally registered products, falls under the TGA black triangle safety monitoring scheme.
Moderna begins vaccine booster trials as evidence of “waning immunity” emerges
In a January 25, 2021 press release Moderna announce the “waning immunity” potential of their COVID-19 vaccine against mutating SARS-CoV-2 variants, in particular to the South African variant B.1.351 which is later called Beta. [1]
“Out of an abundance of caution and leveraging the flexibility of our mRNA platform, we are advancing an emerging variant booster candidate against the variant first identified in the Republic of South Africa into the clinic to determine if it will be more effective to boost titers against this and potentially future variants.” said Stéphane Bancel, Chief Executive Officer of Moderna, confirming talk from earlier in the month about boosters.
Moderna announced they will:
- First, “test an additional booster dos0 “of its current COVID-19 Vaccine (mRNA-1273) to study the ability to further increase neutralizing titers against emerging strains beyond the existing primary vaccination series.
- Second, they would begin “an emerging variant booster candidate (mRNA-1273.351) against the B.1.351 variant…into preclinical studies and a Phase 1 study in the U.S. to evaluate the immunological benefit of boosting with strain-specific spike proteins.
- Moderna expects either one will “further boost neutralizing titers in combination with all of the leading vaccine candidates.”
COVID-19 vaccines won’t stop infection or transmission of SARS-CoV-2
By January 26, 2021, it was already being talked about by public health experts, that the newly rolled-out COVID-19 vaccines will likely NOT prevent infection but more importantly they don’t know if it will stop community transmission of the SARS-CoV-2 virus, the exact justification for mandating vaccines!
The vaccine trials were only designed to assess clinical disease (COVID-19) not prevention of infection or assessing their ability to stop transmission.
The argument supporting vaccination becomes that the vaccine will reduce viral load, and thus assumed to reduce community transmission. But “asymptomatic” individuals already have reduced viral load, if anything viable virus at all, and the already infected produce natural, long-lasting protection.
CDC begins defining a “vaccine breakthrough case” as “vaccine failure” becomes obvious
On January 27, 2021 the CDC (in an email obtained under Freedom of Information) defined a COVID-19 “vaccine breakthrough case” as
“a patient who has SARS-CoV-2 RNA or antigen detected on a respiratory specimen collected [greater than or equal to] 7 days after completing the primary series of an FDA-authorized SARS-CoV-2 vaccine” [1, 2]
Though in retrospect, we learn CDC’s Dr Fisher as early as December 21, 2020, only 7 days after rollout begins, he was directed by a superior “to start working on a protocol to evaluate COVID vaccine failures or breakthrough cases.” Also on January 30, 2021, CDC Director, Dr. Rochelle Walensky, began planting concern about virus variants being a “growing threat” of escaping the protection of vaccines.
On January 27, 2021 at the CDC a 1-page internal document about “vaccine failure” was being distributed by CDC medical officer Dr. Thomas Clark the Epoch Times reveals, but under FOIA it is fully redacted.
On February 2, 2021 in an email, the CDC’s Vaccine Breakthrough Case Investigation Team, led by Dr Fisher and part of the COVID-19 Vaccine Taskforce, alters the definition of a breakthough case to be least 14 days post the completion of a primary dose series of injections. This instantly eliminates cases and provides an inflated and misleading view of vaccine effectiveness. [3]
“A US. resident who has SARS-CoV-2 RNA or antigen detected on a respiratory specimen collected [greater than or equal to] 14 days after completing the primary series of an FDA-authorized COVID-19 Vaccine.”
On February 4, 2021 the CDC communicated with US States on how to count vaccine breakthrough cases and which to exclude!
It wasn’t until April 15, 2021 that the CDC began reporting vaccine Breakthrough Cases, which included some who were hospitalised and died. [4, 5]
On May 1, 2021 the ” CDC transitioned from monitoring all reported vaccine breakthrough cases to focus on identifying and investigating only hospitalized or fatal cases due to any cause”. [6, 7]
In an email to the Epoch Times it was claimed the “CDC made the change to the definition of a breakthrough infection time period due to the most current data that showed that the 14-day period was required for an effective antibody response to the vaccines.” At this point the CDC are assuming antibody production is equivalent to “preventing infection”, a theory. They claimed to have wanted to “eliminate cases where exposure [to the virus] happened before the vaccination response [antibody production] would be effective.”
As Dr Harvey Risch points out to Epoch Times, “If the vaccines don’t work for the first 7 or 14 days or increase risk of getting COVID-19 during that period, that is part of what happens when they [the mass vaccination program] are deployed in a population.”
Dr Jay Bhattacharya told The Epoch Times in an email, the CDC should have warned “recently vaccinated vulnerable older people that they were at higher risk for being infected during that period.”
WHO launch a team to investigate SARS-CoV-2 origin
Upon the back of new evidence, the US Secretary of State ,Mike Pompeo, demanded the WHO to launch an investigation into the possibility that the virus was a “accidental” lab leak.
On January 28, 2021, after 2 weeks of quarantine the WHO probe team begin their investigation in Wuhan, China tasked to learn the origins of SARS-CoV-2, this is a little over one year after the virus was discovered in humans.
Part of the investigation team is Peter Daszak from EcoHealth Alliance, whos organisation provides funding to the Wuhan lab for work on bat coronavirus research, a man who is clearly conflicted.
Shi Zhengli-Li who receives funding from EcoHealth Alliance and claimed no lab staff were infected, is the deputy director of the Wuhan Institute of Virology. The Wuhan lab deleted their data.
A cluster of COVID-19 case was found at the Huanan wet market, where bats were not sold, according to a paper sited by US officials in February 2020.
Lab origin is not ruled out!
CDC Director Walensky emails about “vaccine breakthroughs”
On January 30, 2021 (thanks to Freedom of information (FOIA)) the CDC’s Director, Dr Rochelle Walensky, sends an email titled “vaccine breakthroughs” thus knowing that the COVID-19 vaccines were failing to prevent infection, and thus transmission, contrary to what the public health agency promoted to the public. NIH’s Dr Francis Collins and NIAID’s Dr Anthony Fauci also knew. [1, 2, 3, 4]
Mandated COVID-19 vaccines were based on an alleged “community” benefit meaning that they stopped you from being infected with the SARS-CoV-2 virus and because of this you were unable to transfer it to others!
“Yet, at a press briefing on 16 July 2021, referencing Walensky’s statement that Covid had become the “pandemic of the unvaccinated,” White House press secretary Jen Psaki said: “99.5 percent of people who are in the hospital are people who are unvaccinated. During a CNN town hall event on 20 July 2021, President Joe Biden said that vaccines would ensure that people did not get Covid; or if infected, they would not need hospitalisation; and they would not die.”” [3]
WHO: Plan to generate “acceptance and demand for COVID-19 vaccines”
On January 31, 2021 , now that COVID-19 vaccines were available the WHO and UNICEF needed a plan for how they were going to generate “acceptance and demand for COVID-19 vaccines”. Providing a “Demand Planning Tool” for countries to use “when preparing to introduce COVID-19 vaccines”. [1]
The spreadsheet included:
- Social data collection – Risk Communication and Community Engagement (RCCE)
- Regular Advocacy, Communication and Social Mobilization (ACMS) meetings
- Implementation of mass media, social media plan – Information, Education and Communication (ICE) and public service announcements (PSA), social media boosting
- Social media monitoring and misinformation management – mitigate rumours – critical to maintain public trust in COVID-19 vaccines
- Crisis communication for any possible Adverse Event Following Immunization (AEFI) using Standard Operating Procedures (SOPs)
- Stakeholder and Community engaement programs – target community leaders to deliver Inderpersonal communication (IPC)
- Capacity building – with routine immunization implementation
- Monitor and evaluate effectiveness of communication activities
China: SARS-CoV-2 possibly circulating in parts of the world as early as Sept-Oct 2019
On February 2, 2021 China’s Foreign Ministry spokesperson Wang Wenbin “expressed the hope” that the US government would invite the World Health Organisation (WHO) experts to carry out novel coronavirus source tracing research in the United States. This follows the recent, January 28, 2021 launch by the WHO of a team to investigate the virus origins in China.
Wang noted the reason being that SARS-CoV-2 antibodies were found by the US CDC in blood donations collected that had been collected in December 2019, before the country’s first official case on Jan 21, 2020. There were allegedly “many clues, reports and studies” that indicate the virus had spread to many parts of the world as early as the second half of 2019.”
Additional intel to support Sep-Oct 2019 potential virus releases/escape/spread:
- The World Military Games were held in Wuhan, China October 2019,
- US Intel: China paper suggested Sept 2019 may have been the start
- Wuhan Institute of Virology delete their bat virus genetic sequence database on Sept 12, 2019
- China conducts a novel coronavirus simulation in Wuhan on Sept 18, 2019
- As early as Sept 3, 2019 SARS-CoV-2 antibodies found in blood samples in Italy
- Anonymous prediction on Sept 4, 2019 makes “prophetic” claim of “major event” coming
WHO: the vaccine may not stop transmission of SARS-CoV-2
On February 5, 2021 the WHO’s released their interim position paper and stated “there are still critical unknowns regarding the efficacy of vaccination in reducing transmission”, and that “people who are vaccinated should not be exempt from complying with other travel risk-reduction measures.” [1]
Current “scientific unknowns… concerning the effectiveness of COVID-19 vaccines”:
- efficacy in preventing disease
- ability of limiting transmission including for new variants
- duration of protection
- timing of booster doses
- whether vaccination offers protection against asymptomatic infection
- age and population groups that should be prioritized for vaccination
- how long before travel vaccines should be offered
- possible exemption of people who have antibodies against SARS-CoV-2
For vaccines that were sold as 95% effective (Pfizer), 94.1% effective (Moderna), 2 doses 90% effective (AstraZeneca) – But what does “effective” mean?
Japan approves first COVID-19 vaccine – Pfizer-BioNTech
Japan’s Ministry of Health, Labor and Welfare gave the first “fast-track approval ” to Pfizer-BioNTech COVID-19 vaccine on Sunday February 14, 2021 in ages 16 years and older. Stated as “95 percent effective at preventing symptoms of COVID-19”. [1, 2]
On Wednesday February 17, 2021 it began rollout first with 40,000 healthcare workers who will receive two shots to be administered three weeks apart. “Of the initial group of health workers, 20,000 will participate in a study to track side effects potentially caused by the vaccine.”
“A further 3.7 million front-line health workers are to begin receiving the vaccine in March, followed by 36 million people aged 65 or older from April” 2021. ” People with pre-existing conditions such as diabetes or heart disease and those working at elderly care facilities will come next, and then finally the general population.”
- COVID-19 vaccinations began 6 months out from the start of the 2021 Tokyo Olympics and Paralympics
- In 2020 Japan had no excess death above expected.
- By April 21, 2021 – Japan PM discussed receiving 50 M additional Pfizer doses, on top of existing agreements for 144 M doses – total 194 M doses, enough for 97 M people.
- By May 2023 Japan’s CV19 vaccine dose orders totalled: Pfizer 194M, Moderna 100M, Oxford/AstraZeneca 120M, Novavax 150M, of that 564M total, 10.24M doses were donated to other countries. This includes booster shots. With Japan’s population in 2021 as 125.6M, the remaing doses equate to 4.4 shots for every single person.
Timeline pages:
1800s | 1900-1945 | 1946-1979 | 1980-1999 | 2000-2015 | 2016-2018 | 2019 | 2020 | 2021 | 2022 | 2023 | 2024 | 2025
