Novavax COVID-19 Vaccine by Novavax, Inc. contains SARS-COV-2 (COVID-19) vaccine, subunit, recombinant spike protein-nanoparticle+Matrix-M1 saponin-based adjuvant, preservative free, 0.5mL dose. [CDC]
The adjuvant which contains saponin extracts from the bark of the Soapbark tree is native to Chile. The adjuvant is used to stimulate the immune response towards the spike antigen when injected. The spike protein in this vaccine is produced in insect cells. This vaccine also contains Polysorbate 80, which is linked to adverse reactions.
Novavax, Inc. (Nasdaq: NVAX) is a clinical-stage biotechnology company, creating novel vaccines to address a broad range of infectious diseases worldwide, including H1N1, using advanced proprietary virus-like particle (VLP) technology. The Company produces these VLP based, potent, recombinant vaccines utilizing new and efficient manufacturing approaches.
Unlike the mRNA vaccines, each dose delivers a set amount of protein, there is no production off-button for the mRNA technology injections.
Novavax is a small Maryland biotech company, founded in 1987, yet it had NEVER brought a vaccine product to market in it’s long 33-year history, just like BioNTech and Moderna.
The company received $1.8 billion in US taxpayer funding from Operation Warp Speed, and struggled to build a manufacturing base from scratch and its clinical data came much later than Pfizer or Moderna. Before this CEPI awarded US$388 million for Phase 1 and ongoing trials [see early 2020 below].
The company uses proprietary lipid vesicle encapsulation technologies. Some Novavax history.
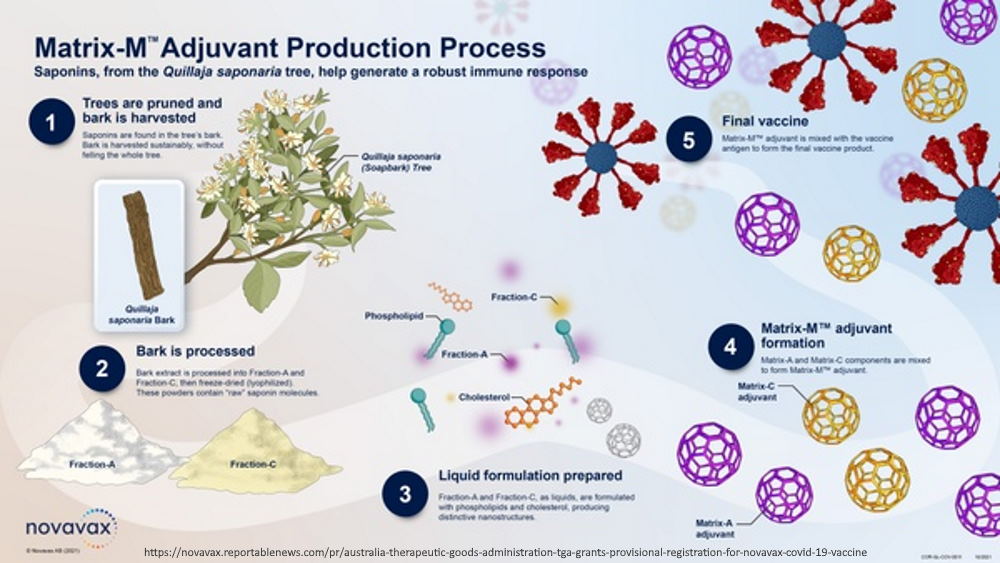

TGA – Australia – NUVAXOVID
Novavax related articles and links in reverse chronological order
This page is continuously being up dated
2023
September 25, 2023 – Jikkyleaks Twitter(X): all the mechanisms for DNA integration that were found in the Pfizer vaccine in the #plasmidgate investigations by @Kevin_McKernan and @P_J_Buckhaults are also potentially present in Novavax if there is plasmid or BCV contamination. – THREAD, More on whats in the vaccine – HERE
- Novavax “added saponin which is a transfectant medium” – TWEET
May 3, 2023 – Excess Deaths AU Substack: Australia’s Novavax Vaccine Contracts – READ
- Australia Novavax Advance Purchase Agreement (Public Redacted) – Via Knowledge Ecology International – Dated December 31, 2020 – PDF
- Australia’s Jane Halton, chairperson of CEPI who invested in Novavax’s vaccine, also reviews Australia’s vaccine contracts! – REF
- “8.5 Regulatory Assistance: Customer (The Australian Government) will …support Novavax in obtaining Regulatory Approval for the Vaccine …including accelerated Regulatory Approval processes…”

January 26, 2023 – FDA | VRBPAC meeting to Discuss Future Vaccination Regimens Addressing COVID-19 – WATCH, Meeting minutes links – HERE, PRESS, Federal Register – READ Novavax presentation – SLIDES looking at Homologous and Heterologous Boosting (mixing technologies)
[COVID-19 was all about establishing a vaccine PLATFORM]

2022
November 9, 2022 – PRESS RELEASE: Novavax Nuvaxovid™ COVID-19 Vaccine Authorized in the United Kingdom for Use as a Booster in Adults – Nuvaxovid™ (NVX-CoV2373) COVID-19 vaccine▼ – READ
- Conditional Marketing Authorization (CMA) for Novavax “as a homologous and heterologous booster dose after the primary series of Nuvaxovid (six months) or of an mRNA or adenoviral vector vaccine” – [meaning mix and match is ok]
- “”Our protein-based vaccine, developed using an innovative approach to traditional technology, may have a prominent role to play in COVID-19 boosting,” said Stanley C. Erck, President and Chief Executive Officer, Novavax.”
- “The MHRA decision was based on data from Novavax’ Phase 2 trial conducted in the U.S. and Australia (1,283 participants), from a separate Phase 2 trial conducted in South Africa (4,404 participants), and from the U.K.-sponsored COV-BOOST trial (2,878 participants)…”
November 8, 2022: PRESS RELEASE: Novavax Phase 3 COVID-19 Omicron Trial Supports the Continued and Future Use of Novavax Prototype Vaccine as a Booster – READ, ARCHIVE
October 19, 2022 – PRESS RELEASE: U.S. FDA Grants Emergency Use Authorization for Novavax COVID-19 Vaccine, Adjuvanted as a Booster for Adults – READ, ARCHIVE
- Novavax’ vaccine is also available for use as a booster in adults aged 18 and older in the European Union, Japan, Australia, New Zealand, Switzerland, and Israel.
October 13, 2022 – PRESS RELEASE: Novavax COVID-19-Influenza Combination Vaccine Candidate Induced Antibody and T-Cell Responses Against SARS-CoV-2 and Homologous and Heterologous Influenza Strains – READ
September 25, 2022 – News Australia: Novavax appeals to ATAGI to change advice as vaccine uptake falls behind – Novavax was hailed as the fix to Australia’s low inoculation rate and “the vaccine for anti-vaxxers” – But of the 51 million Novavax doses purchased by the Commonwealth and 13.3 million delivered, only 218,000 shots have been administered (less than 1%) — leaving millions destined for the bin as their expiry date looms. – READ, Rebel News – READ
September 12, 2022: PRESS RELEASE: Novavax Nuvaxovid™ COVID-19 Vaccine Granted Expanded Conditional Marketing Authorization in the European Union for Use as a Booster for Adults Aged 18 and Older – READ
September 6, 2022 – The Mainichi – Man dies after receiving Novavax COVID shot in Japan – 29 year old died with in 24 hours of second shot – “but a causal relation remains unclear.” – READ
August 19, 2022 – PRESS RELEASE: U.S. FDA Grants Emergency Use Authorization for Novavax COVID-19 Vaccine, Adjuvanted for Adolescents Aged 12 Through 17 – READ
- “The FDA EUA decision was based on data from the ongoing pediatric expansion of the Phase 3 PREVENT-19 trial of 2,247 adolescents aged 12 through 17 years across 75 sites in the U.S.”
August 18, 2022 – Dr Pierre Kory Substack: What to Know Before Deciding to Take The Novavax Injection – “Before any medical intervention, but especially in the case of a novel or barely tested one, a long standing practice of medical ethics is that informed consent must be obtained.” – READ
August 15, 2022: PRESS RELEASE: Novavax Submits Application to the U.S. FDA for Emergency Use Authorization for Novavax COVID-19 Vaccine, Adjuvanted as a Booster in Adults Aged 18 and Older – READ
August 3, 2022 – Reuters: EU says Novavax COVID shot must carry heart side-effect warning – READ, ARCHIVE, GETTR
July 19, 2022 – PRESS RELEASE: U.S. CDC Advisory Committee [ACIP] Unanimously Recommends Novavax COVID-19 Vaccine, Adjuvanted as a Primary Series for Individuals Aged 18 and Older – READ
July 15, 2022 – CNBC: Novavax’s new Covid vaccine is perfect for people scared of mRNA tech—but it won’t win over the unvaccinated – READ
July 15, 2022 – CNBC: FDA authorizes Novavax Covid vaccine for adults as the first new shots in U.S. in more than a year – READ
- The authorisation was delayed as the company changed it’s manufacturing process.
- Novavax received receiving $1.8 billion in taxpayer funding from Operation Warp Speed.
- The CDC still needs to sign off on Novavax’s vaccine before pharmacies and other health-care providers can start administering shots.
July 14, 2022 – Epoch Times: Top Regulator: ‘Severe Allergic Reaction’ a Side Effect of New COVID-19 Vaccine – READ
July 13, 2022 – PRESS RELEASE: U.S. FDA Grants Emergency Use Authorization for Novavax COVID-19 Vaccine, Adjuvanted for Individuals Aged 18 and Over – Novavax’ vaccine is the first protein-based COVID-19 vaccine authorized in the U.S. – READ
July 13, 2022 – FDA: Coronavirus (COVID-19) Update: FDA Authorizes Emergency Use of Novavax COVID-19 Vaccine, Adjuvanted – READ
July 13, 2022 – FDA: Novavax COVID-19 Vaccine, Adjuvanted – FACT SHEETS
July 11, 2022 – PRESS RELEASE: U.S. Government Secures 3.2 Million Doses of Novavax COVID-19 Vaccine – READ
June 16, 2022 – Johns Hopkins University HUB: What you need to know about the Novavax vaccine – Vaccine expert William Moss discusses its strengths and weaknesses ahead of its potential emergency use authorization – READ
June 14, 2022 – GP NEWS: Novavax receives COVID-19 booster approval – The protein-based vaccine is the third to gain TGA approval as a booster in Australia, following Pfizer and Moderna – READ
June 13, 2022 – PRESS RELEASE: Novavax COVID-19 Vaccine Nuvaxovid™ Provisionally Registered in Australia as a Booster in Individuals Aged 18 and Over – READ
- The provisional registration was based on data from Novavax’ Phase 2 trial conducted in Australia…
June 9, 2022 – Australia’s Therapeutic Goods Administration (TGA) grants Provisional Approval for NUVAXOVID as a booster in individuals aged 18 years and older – READ, READ
June 9, 2022 – CNBC: FDA decision on Novavax’s Covid shots could be delayed to review changes in manufacturing – READ
- Novavax informed the Food and Drug Administration of changes to its manufacturing process on June 3, days before the agency’s committee was scheduled to review the vaccine.
- Novavax asked the FDA to authorize its two-dose vaccine in late January 2022
- Novavax is already authorized in more than 40 countries, including Australia, Canada and the European Union.
June 7, 2022 – PRESS RELEASE: FDA Advisory Committee [VRBPAC] Recommends Emergency Use Authorization of Novavax COVID-19 Vaccine for People Aged 18 Years and Older – READ
July 25, 2022 – Meryl Nass Substack: Novavax vaccine contains 1 mcg of insect (the fall armyworm) and baculovirus proteins and a bit of their DNA too, which is injected into you with each dose – And that’s before we consider the Matrix-M novel adjuvant it contains – READ
July 19, 2022 – CDC: Meryl Nass “live blog comments” on the ACIP meeting “Clown Show”: Myocarditis Risk Update, Novavax Safety + More — READ
- The “Novavax vaccine was being rolled out because it could be marketed as a “more traditional” vaccine” – REF
- U.S. already purchased 3.2 M doses of Novavax C19 vaccine – pressuring the committee to approve it!
May 22, 2022 – Epoch Times: Novavax’s 22 Percent Post-Earnings Plunge: 5 Factors That Took Toll – READ
April 29, 2022 – PRESS RELEASE: FDA Announces Vaccines and Related Biological Products Advisory Committee [VRBPAC] Review of Novavax’ COVID-19 Vaccine – READ
- NVX-CoV2373 would be the first protein-based COVID-19 vaccine to be reviewed by VRBPAC in the U.S.
April 1, 2022 – Epoch Times: Novavax Asks EU Regulator to Clear COVID Vaccine for Teens )European Medicines Agency) – READ
March 15, 2022 – Epoch Times: Novavax’s COVID Vaccine Rollout in European Union Off to a Slow Start: Data – READ
March 2, 2022 – Epoch Times: Novavax Approved as Booster Alternative for Australians 18 and Over – READ
March 2, 2022 – The Australian Technical Advisory Group on Immunisation (ATAGI) has recommended the use of the Novavax vaccine as a booster in Australians aged 18 and over where an mRNA vaccine is not suitable – READ
February 23, 2022 – Epoch Times: Australia Approves Moderna Vaccine For 6 to 11 year olds – READ
February 19, 2022 – Epoch Times: Novavax COVID 19 Vaccine Gets Approval for Use in Singapore – READ
February 17, 2022 – Epoch Times: Novavax COVID 19 Vaccine Gets Approval for Use in Canada – READ
February 14, 2022 – Epoch Times: Novavax COVID 19 Vaccine Gets Approval in Switzerland– READ
February 11, 2022 – Epoch Times: Novavax Rollout in Australia to Begin One Week Earlier – READ
February 10, 2022 – Epoch Times: Novavax Underdelivers on COVID-19 Vaccine Promises – It has delivered just a small fraction of the 2 billion COVID-19 shots it plans to send around the world in 2022 – READ
February 10, 2022 – NEJM: Efficacy and Safety of NVX-CoV2373 in Adults in the United States and Mexico – Novavax et al – READ
February 3, 2022 – Epoch Times: United Kingdom Conditionally Approves Its First Protein-Based COVID-19 Vaccine, From Novavax – READ
February 3, 2022 – Epoch Times: Novavax Passes First Hurdle for New Zealand Vaccine Rollout – Regulator grants provisional approval – READ
January 31, 2022 – Epoch Times: Novavax has formally submitted a request to FDA seeking Emergency Authorization for COVID-19 Vaccine (NVX-CoV2373) – READ, OTHER
January 31, 2022 – PRESS RELEASE: Novavax Submits Request to the U.S. FDA for Emergency Use Authorization of COVID-19 Vaccine – READ
- NVX-CoV2373 demonstrated overall efficacy of ~90% in PREVENT-19 clinical trial conducted during the emergence of variant strains”
- “Novavax conducted two pivotal Phase 3 clinical trials: PREVENT-19 which enrolled approximately 30,000 participants in the U.S. and Mexico and published results in the New England Journal of Medicine (NEJM) and a trial with almost 15,000 participants in the U.K. which was also published in NEJM. In both trials, the vaccine demonstrated efficacy with a reassuring safety profile.”
January 19, 2022 – TGA provisionally approves Biocelect Pty Ltd’s (on behalf of Novavax Inc) COVID-19 vaccine NUVAXOVID – READ, TGA provisional reg – WEBSITE
- This protein vaccine is provisionally approved and included in the Australian Register of Therapeutic Goods (ARTG) for active immunisation to prevent COVID-19 in individuals 18 years of age and older. It is recommended that the vaccine is given in 2 doses administered 3 weeks apart.
- The product has been under evaluation since February 2021 – READ
January 19, 2022 – Epoch Times: Australia Approves Novavax COVID-19 Vaccine and New COVID Oral Drug Treatments (Paxlovid and Lagevrio) – READ
January 19, 2022 – PRESS RELEASE: Australia Therapeutic Goods Administration (TGA) Grants Provisional Registration for Novavax COVID-19 Vaccine – READ
- The approval for provisional registration by the TGA is based on the totality of preclinical, manufacturing and clinical trial data submitted for review. This includes two pivotal Phase 3 clinical trials…”
2021
December 31, 2021 – PRESS RELEASE: Novavax Submits Final Data Packages to U.S. FDA as Prerequisite to Emergency Use Authorization Application Request for COVID-19 Vaccine – READ
December 22, 2021 – Epoch Times: WHO Experts Recommend Third Dose of Novavax COVID Vaccine for People With Health Issues – READ
December 22, 2021 – PRESS RELEASE: Novavax Announces Initial Omicron Cross-Reactivity Data from COVID-19 Vaccine Booster and Adolescent Studies – READ
December 20, 2021 – Epoch Times: European Union Authorizes COVID-19 Vaccine From US-Based Novavax the first protein-based vaccine – enabling its use in all 27 member-states- READ
December 20, 2021 – WHO: Background document on the Novavax (NVX-CoV2373) vaccine against COVID-19 – READ
December 20, 2021 – PRESS RELEASE: World Health Organization Grants Second Emergency Use Listing ([EUL] for Novavax COVID-19 Vaccine – READ, EUL – HERE
- Nuvaxovid™ COVID-19 Vaccine (SARS-CoV-2 rS [Recombinant, adjuvanted]) listed for emergency use by the WHO – READ, ARCHIVE
December 17, 2021 – CEPI Press: CEPI statement: CEO welcomes Emergency Use Listing for NVX-CoV2373 – READ
- March 2020, CEPI provided up to US $4 million for Novavax to use their recombinant protein nanoparticle technology to develop a vaccine candidate against COVID-19.
- May 2020 – second investment: provided up to US $384 million to support preclinical studies, Phase 1 and Phase 2 clinical trials. The additional funding also supported the transfer of NVX-CoV2373 technology to manufacturing partners in different geographical regions for large-scale production of the vaccine.
December 17, 2021 – WHO lists 9th COVID-19 vaccine for emergency use with aim to increase access to vaccination in lower-income countries – emergency use listing (EUL) for NVX-CoV2373 – READ, ARCHIVE
- “CovovaxTM is a subunit of the vaccine developed by Novavax and the Coalition for Epidemic Preparedness Innovations (CEPI).
- It requires two doses and is stable at 2 to 8 °C refrigerated temperatures. The vaccine uses a novel platform and is produced by creating an engineered baculovirus containing a gene for a modified SARS-CoV-2 spike protein.
- The originator product produced by Novavax, named NuvaxovidTM, is currently under assessment by the European Medicines Agency (EMA).”
November 27, 2021 – Epoch Times: US-Based Company Developing Vaccine That Targets Newly identified Omicron COVID-19 Variant – READ
November 9, 2021 – Becker Hospital: Why hasn’t Novavax’s COVID-19 vaccine been approved yet? – READ
October 29, 2021 – PRESS RELEASE: Novavax Files for Provisional Approval of its COVID-19 Vaccine in Australia – READ
- “Australia has played a pivotal role in the Phase 1 and Phase 2 clinical trials supporting the development of NVX-CoV2373, the company’s recombinant nanoparticle protein-based COVID-19 vaccine with Matrix-M™ adjuvant.
- Additional booster trials and a Phase 1/2 trial for a combination vaccine using Novavax’ seasonal influenza and COVID-19 vaccine are underway in Australia.”
October 27, 2021 – PRESS RELEASE: Novavax Files for Authorization of its COVID-19 Vaccine in the United Kingdom – READ
October 20, 2021 – Politico: Novavax’s manufacturing challenges threaten global vaccine supply – READ
September 23, 2021 – NEJM: Safety and Efficacy of NVX-CoV2373 Covid-19 Vaccine – Novavax et al – READ (Preprint June 30, 2021)
August 30, 2021 – PRESS RELEASE: Novavax Statement on CDC Guidance Update for COVID-19 Clinical Trial Participants – READ
July 19, 2021 – KHN: Novavax’s Effort to Vaccinate the World, From Zero to Not Quite Warp Speed – READ
July 15, 2021 – The New American: COVID Criminals WILL Be Held Accountable, Says Atty. Dr. Fleming in Warning Against Novavax – WATCH, BACKUP
- Dr. Fleming noted that the spike protein, which these shots are causing to be produced in one way or another, is the actual bio-weapon being used against the population.
June 15, 2021 – Epoch Times: Novavax Says Could Offer COVID-19 Vaccine Together With Flu Shot for Boosters – READ
- Novavax said that co-administering its protein-based COVID-19 vaccine with an approved flu shot may be a “viable immunization strategy” in the future, based on preliminary findings from a recent study.
June 14, 2021 – CNN: Novavax says Covid-19 vaccine shows 90.4% overall efficacy in US/Mexico Phase 3 trial – WATCH & READ
- “The study launched in December and enrolled 29,960 adults across 113 sites in the United States and six sites in Mexico. Some of the participants were given a placebo and some were administered two doses of the Novavax vaccine 21 days apart.”
June 14, 2021 – Elevate: Novavax’s Prevent-19 lives up to its name – But with many Western markets already saturated, it is worth wondering how important Novavax’s Covid-19 jab will become. – READ
May 14, 2021 – The Highwire: IS COVID-19 A BIO-WEAPON? with Dr Fleming – WATCH
May 13, 2021 – Bloomberg Markets: Novavax Vaccine in ‘Good Shape’ for Approval Filing: CEO – – Novavax Inc. CEO Stan Erck says manufacturing challenges are behind the delay in the company’s plan to file for authorization of its Covid-19. – WATCH, Seeking Alpha – READ
February 17, 2021 – WSJ (source): Insiders at Covid-19 Vaccine Makers Sold Nearly $500 Million of Stock Last Year – READ, WSJ – READ
- “At Novavax Inc., executives sold more than $40 million of their shares after the company’s vaccine hit milestones in August and September.”
- “Novavax executives sold $23 million of shares in August a day after the company announced the beginning of the second phase of its clinical trial.”
- “In late September, executives at Novavax sold another $19 million in shares days after entering phase three of the clinical trial for its vaccine candidate.”
- “Moderna and Pfizer insiders who set up preset trading plans last year all had cooling-off periods of less than three months. Novavax insiders didn’t disclose dates for the adoption of their preset trading plans.”
January 28, 2021 – Epoch Times: Novavax Says Vaccine 89.3 Percent Effective in preventing COVID-19 in UK Trial, but Less effective in South Africa where a different virus variant is more prevalent – READ
January 22, 2021 – SMH | Australia: Sydney startup to help vaccine hopeful Novavax roll out jab – US vaccine maker Novavax has selected a Sydney Biocelect which was founded in 2014 – READ
January 19, 2021 – TGA grant Provisional Determination for Novavax – which means the TGA will assess to see if the product can move through the Provisional Registration pathway – TGA, REF, [Note the media claim this as the “registration” process but that is technically not correct and deceptive – provisional is a separate pathway]
January 7, 2021 – PRESS RELEASE: Novavax Finalizes Agreement with Commonwealth of Australia for 51 Million Doses of COVID-19 Vaccine – READ , IMAGES, [Australia’s total population is ~27 million!, Pfizer, Moderna & A/Z vaccine also purchased!!] – Contract confirms 51 million, 10 million additional – PDF (pg 31)
2020
December 29, 2020 – CNN: Novavax coronavirus vaccine becomes fifth to begin Phase 3 trials in United States – READ
December 28, 2020 – PRESS RELEASE: Novavax Announces Initiation of PREVENT-19 Pivotal Phase 3 Efficacy Trial of COVID-19 Vaccine in the United States and Mexico – ARCHIVE
- “PREVENT-19 (the PRE-fusion protein subunit Vaccine Efficacy Novavax Trial | COVID-19) is a randomized, placebo-controlled, observer-blinded study to evaluate the efficacy, safety and immunogenicity of NVX-CoV2373 with Matrix-M in up to 30,000 subjects 18 years of age and older compared with placebo.”
July 7, 2020 – NY Times: U.S. Will Pay $1.6 Billion to Novavax for Coronavirus Vaccine – ARCHIVE
July 7, 2022 – HHS PRESS RELEASE: HHS, DOD Collaborate with Novavax to Produce Millions of COVID-19 Investigational Vaccine Doses in Commercial-Scale Manufacturing Demonstration Projects – ARCHIVE
- “The U.S. Department of Health and Human Services (HHS) and Department of Defense (DoD) today jointly announced a $1.6 billion agreement with Novavax, Inc. of Gaithersburg, Maryland, to demonstrate commercial-scale manufacturing of the company’s COVID-19 investigational vaccine. By funding this manufacturing effort, the federal government will own the 100 million doses of investigational vaccine expected to result from the demonstration projects.”
May 11, 2020 – PRESS RELEASE: CEPI extends collaboration with Novavax to advance development and manufacture of COVID-19 vaccine – READ
- CEPI to provide up to an additional US$384 million funding for accelerated development of phase 1 and 2 clinical trials and large-scale manufacture of Novavax’ NVX-CoV2373 vaccine candidate, taking total investment in NVX-COV2373 to US $388 million
- A phase 1 clinical trial will begin this month in Australia, followed by phase 2 clinical trials in multiple countries
April 16, 2020 – News Wire | Nucleus Network Australia: Novavax to commence COVID-19 vaccine trial with Nucleus Network – Phase 1 clinical trials to begin at Melbourne and Brisbane clinics – rapid testing with first-in-human trials – READ, BioSpace – READ, Nucleus network – WEB
- Nucleus Network was established by the Victorian government in 2003 – READ
March 10, 2020 – PRESS RELEASE: CEPI expands investment in COVID-19 vaccine development – investing $4.4 million in partnering agreements with Novavax, Inc., to enable preparations for phase 1 trials – READ
- “The investments announced today are a result of a recent global call for proposals that CEPI issued in early February, which invited funding applications for proven vaccine technology that could be used to rapidly develop a vaccine against the new coronavirus, and most importantly at scale and with the necessary equitable access provisions.”
- “CEPI and Novavax agree on the importance of global equitable access to vaccines produced out of our partnership. Under the terms of the agreement it is anticipated that vaccines will be procured and allocated through global mechanisms now under discussion as part of the Access to COVID-19 Tools (ACT) Accelerator, an international initiative launched by the WHO and global leaders last month.”
2011
February 28, 2011 – HHS NEWS: HHS awards contracts to develop new flu vaccine technology – contracts for new types of vaccines – Novavax $97 million over the first three years up to $179.1 million if extended – ARCHIVE
- The 2009 H1N1 pandemic demonstrated the need for technologies that can provide vaccines more rapidly,” [The Justification!]
- Under its contract, Novavax is to develop new technology to produce vaccines using insect cells to express influenza proteins and create virus-like particles that stimulate a strong immune response in humans. [sound familiar for coronavirus in 2020?]
- Curiously – the other award totalling $196.6 million went to VaxInnate – a start-up developing vaccines manufactured using bacterial expression/fermentation and are based on its potentially fast, Toll-like receptor (TLR) technology platform. The company has finacial ties to NIAID, Wellcome Trust and Oxford, and venture capital firms REF
- Dr Seth Berkley is on the Board of Directors – who would become the CEO of GAVI in 2020 – REF
- They were “proud partners” with BARDA ($196 million over five years) and NIH ($2.2 million) and Emergent Biosolutions
- It appears VaxInnate never brought a product to market – REF, by 2014 their clinical trial looks abandoned – REF
- They close business 5 years after the near $200 million NHS grant, in January 2017.




