Remdesivir is “a nucleotide analogue prodrug that inhibits viral RNA polymerases,” (an intravenous anti-viral drug) made by Gilead Sciences that was pulled from an Ebola trial due to it increasing mortality. [2]
Dr Bryan Ardis in early 2020 looked into remdesivir, on the back of losing his father-in-law to this very drug in February 2020, and revealed to the world that the NIH’s promotion of remdesivir as the only allowed hospital treatment for COVID-19 was in fact increasing mortality – a death that was certified as a COVID-19 death! Dr Ardis provides his overview of remdesivir findings at the Grand Jury inquiry – HERE.
Australia’s TGA granted provisional registration for remdesivir (Veklury) in early 2020. [1]
Giliead Sciences – the company that makes remdesivir:
- Gilead Sciences website – HERE, ARCHIVES
- Gilead Press Release – 2020 ARCHIVES
- Gilead Sciences Ongoing Response To COVID-19 – ARCHIVES
Hospital Protocols force remdesivir onto patients – what hospitals are doing and how to protect yourself and your loved ones
Following are links to aid with your own research into Remdesivir
2024
March 9, 2024 – Geoff Pain Substack: Remdesivir Deaths – was Endotoxin a factor? – READ
- Chemical structure of Remdesivir has organophosphate and cyanide groups but looking at symptoms, could Endotoxin contamination, as found in India, be the caused deaths?
February 2, 2024 – CHD Bus Stories: “They’re Still Killing With Remdesivir” – Nancy Wilson was “forced” to get the COVID-19 shot under mandates, she then tested positive while in hospital, multiple staff asked her to take remdesivir. She declined and took ivermectin instead. Find out how the doctors reacted – WATCH
Jan-Feb 2024 – CHD Bus Stories: Loved one’s killed by COVID-19 hospital protocols, usually involving remdesivir (a small collection of stories):
- My Wife Was Killed By Hospital Covid Protocol – Clyde “Rick” Stephenson – WATCH
- They Killed My Wife In The Hospital She Worked At – Mark Hartshorn’s wife, Jill – WATCH
- My Wife Survived Covid Hospital Protocol – Richard‘s story – WATCH
- My Daughter Snuck Ivermectin Into The Hospital – Micheal Murphy‘s story – WATCH
- Dennis tells the story of his friend, Daniel Freeman, who passed away in the hospital while being treated for the virus – WATCH
- My Husband Was Killed By Hospital Covid Protocol – Juliana‘s story – WATCH
- My Mother Died In Hospital During Covid – Kelly warning – WATCH
- My Husband Was Killed by Covid Hospital Protocol – Cynthia‘s recounts the heart-wrenching details – WATCH
- They Tried To Kill Me – WATCH
- My Momma Was Killed By Hospital Covid Protocol – Kristina Croft – WATCH
- They Killed My Healthy Husband – Jill Smith obtained over 6,000 pages of records, 60 of which listed drugs he was administered – WATCH
- My Husband Survived Hospital Covid Protocol – Robyn‘s story – WATCH
- Michelle Mixa cousin cousin die in the hospital – WATCH
- My 30 Year Old Daughter Chasity Anderson Was Killed By A Hospital Chasit – She was put on remdesivir, labeled ‘DNR,’ isolated from family and put on a ventilator, she did not make it out of the hospital alive – WATCH
- They Killed My Mom With Remdesivir – Crystal shares how her mum went to hospital with abroken hip, they tested for COVID-19 it was positive, she then fell victim to “hospital protocol” for positive test!!! – he mum had “no symptoms, whatsoever,”- WATCH
- I Survived The Hospital Covid Protocol – Valerie‘s story, more than just a lack of informed consent. While a patient, she made known her wishes about treatment and end-of-life care by writing them on her arm, yet Valerie still felt an “overwhelming fear” about her situation – WATCH
- Hospital Covid Protocol Death – In August 2021, when his parents tested positive for COVID. While his vaccinated mother was sent home, his father, who had received no COVID shots, was admitted to the hospital to receive “separate treatment.” – Wayne and Mary‘s story – WATCH
- My Dad Was Killed By Remdesivir – story of Renee’s father – WATCH
- My Husband Ray Was Killed By Covid Hospital Protocol – WATCH
- My Husband Survived Hospital Protocol – as an employee of the pharmaceutical industry she was shocked and surprised – WATCH
- My Dad Was Killed By Hospital Protocol –Andy‘s story – WATCH
- Hospital Hostage Rescue – Within hours of being admitted, Gail Seiler remembers being told by a doctor that she was going to die but that “the only hope” she had for survival “was remdesivir and a vent.” – WATCH
- They Killed My Mom – Elizabeth Kucker‘s story – WATCH
- My Mom Was Killed By Covid Hospital Protocol after 53 days – Genny Rodriguez‘s story, find out what she uncovered from the medical records after the fact. – WATCH
- My Husband Was Killed By Hospital Protocol – Liz recounts Kevin’s tragic death – WATCH
- They Killed My Husband – Vanessa Tilley speaks out – WATCH
- Covid Hospital Protocol Killed My Husband – Lisa Butler shares her husband’s medical records – WATCH
2023
October 18, 2023 – Daily Clout c/ Dr Naomi Wolf: Grace’s Dad (Scott Schara) Tells Heartbreaking Story of His Daughter’s Murder at Hospital – WATCH
- He and his family lost their lovely, ebullient, formerly perfectly healthy 19-year-old daughter Grace at Ascension Hospital after she was admitted with COVID and an unlawful “Do Not Resuscitate” notification was placed, against her family’s will, in her records.
August 9, 2023 – The Dossier Substack by Jordan Schachtel: Fauci successor at NIAID peddled dangerous Remdesivir drug as ‘silver bullet’ against Covid-19-Dr. Jeanne Marrazzo tried to use unsafe antiviral IV drug on every covid hospitalized patient at UAB. – READ
August 9, 2023 – Rounding the Earth Substack: BOMBSHELL: New FOIA Documents Reveal the COVID Pandemic Was a DoD Operation Dating Back to Obama – Jeanne Marrazzo has been queened to replace Fauci as head of NIAID – insights – READ
- July 2020, Marrazzo at University of Alabama called using HCQ as “irresponsible and despicable” – she is funded by Gilead pharma for remdesivir trials!
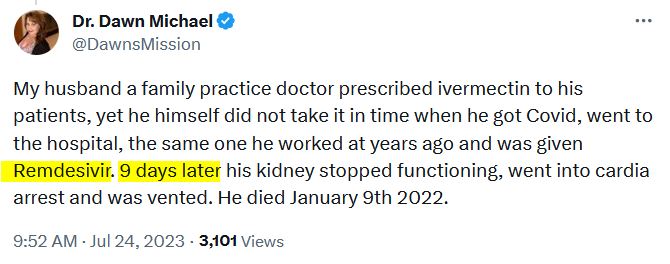
July 24, 2020 – Testimonial – Remdesivir’s typical trail of destruction, knocked out kidneys in 9 days – TWEET , So sorry for your loss Dawn.
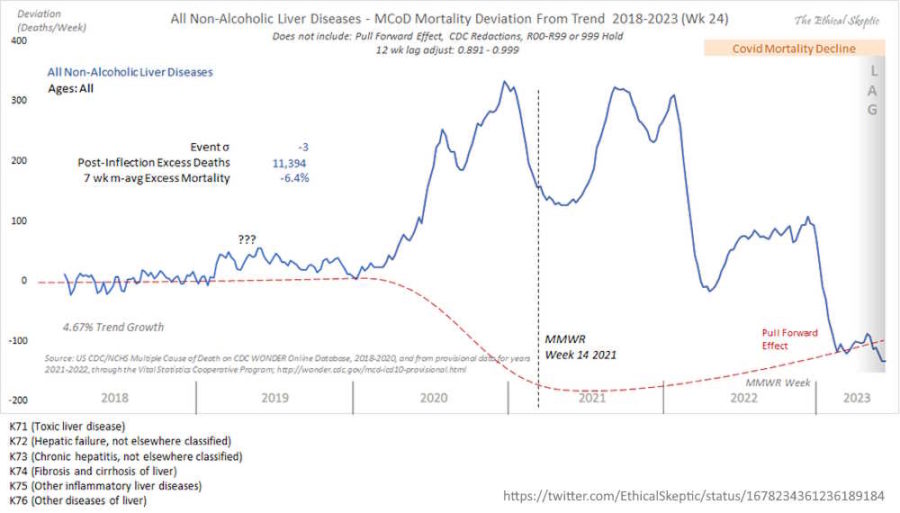
July 10, 2023 – Ethical Skeptic: Those who have made arguments that Remdesivir was deadly, have a data to back their contention as a hypothesis. – THREAD
- Non-Alcohol related Liver Mortality did not arrive with Covid, it arrived with Remdesivir. Now this ICD set has recovered fully to PFE-baseline
May 30, 2023 – Brownstone Institute: Why Are Hospitals Still Using Remdesivir? – Two smoking guns – READ
- “Alas, the federal government insisted that if hospitals wanted to get paid, they had to treat Covid patients with Remdesivir. The fact that this drug was made by their good friends at Gilead Science and everybody was getting rich from the deals they cut had absolutely nothing to do with it, of course. It was all done for love of the people. But just to make sure that Remdesivir could attain its current billion-dollar status, the feds incentivized hospitals with a 20 percent boost to the entire hospital bill of patients treated with Remdesivir. “
- CMS.gov – still paying on Veklury (remdesivir) til September 30, 2023, even though emergency officially ended – ARCHIVE, [January 21, 2022 – FDA granted supplemetal approval remdesivir for COVID-19, April 25, 2022 EUA revoked
- “The 20 largest hospitals enjoyed a 62 percent increase in their combined net assets during those glorious Covid years, providing many top executives with a $10 million salary or more.” Open Books – READ
May 26, 2023 – CHD Th Peoples Testament: COVID Vaccine Injured Police Officer Killed by Remdesivir – guest Karlyn Swoap – WATCH
May 7, 2023 – C’s Newsletter Substack by Charles Wright: The Duality of Steve Kirsch – Remdesivir and Vaccine Adverse Events. – READ
- Steve Kirsch established Covid Early Treatment Fund (CETF) in April 2020,
- He let Rockefeller Philanthropy Advisors manage the fund
- Kirsch’s CETF funded research on several chemical compounds, including Remdesivir.
- Mr. Kirsch also reported on Twitter that he had a friend at Gilead, the patent holder of Remdesivir.
- [Reserve your judgement of Steve Kirsch based on his funding of this product at this point in time (April 2020) I’d suggest he was just trying to find an answer, he explains his early journey on June 2021 podcast with Bret Wienstien and Dr Robert Malone. I place this information here to capture the big picture. Everyone is on a journey of “discovery”!]
March 15, 2023 – The Expose: Remdesivir estimated to have killed 100,000 Americans – READ
2022
October 27, 2022 – The Highwire Ep 291 – REMDESIVIR NIGHTMARE: “I ABSOLUTELY BLAME THE HOSPITAL FOR HIS DEATH” – interview with two widows whose husbands lost their lives while on the same COVID-19 treatment protocol at Beaumont Hospital in Michigan – WATCH, FULL
October 18, 2022 – Nature Communications: Effect of remdesivir post hospitalization for COVID-19 infection from the randomized SOLIDARITY Finland trial – Nevalainen et al – READ
September 26, 2022 – Vigilant Fox: Incentivized to KILL: Prescribe Remdesivir, and We’ll Give You a 20% Bonus – EXCERPT, WATCH, Interview with Steve Kirsch – SOURCE, More Dr Marik – HERE
“The hospital bill, the average, is about $400,000 to $500,000 per COVID patient — PER COVID patient.”
Dr Paul Marik
[So that’s an $80,000 to $100,000 BONUS]
September 22, 2022 – The Highwire Ep 286 – LAWYERS SUE HOSPITALS FOR REMDESIVIR DEATHS [ALARMING] – WATCH, FULL
- The Fauci promoted NIH Remdesivir RCT clinical trail started Feb 21, 2020 enrolling 1100 individuals, it concluded that patients had a “31% better chance of recovering and getting out of the hospital”
- They changed the trial endpoints from reduced mortality, to days in hospital. They unblinded patient and discharged treatment group from hospital early!
- Anyone who came to a hospital and “diagnosed” with COVID-19 were put on remdesivir. Hospitals incentivised, patients restrained to beds with cable ties, drugged with morphine, starved, dehydrated, phone remove and isolated from their family – the average time to death on remdesivir is 9 days! = torture
- Patients threatened if they leave hospital against medical advice, insurance won’t cover hospital cost = blackmail
- Lawyers have thousands of alarmingly similar testimonies across 32 states.
- Some clients received 30-40 different drugs, many contraindicated with remdesivir!
September 7, 2022 – Towards The Light: Remdesivir deaths press conference – Fresno, California – WATCH
August 16, 2022 – Gilead PRESS RELEASE: Veklury® (Remdesivir) Demonstrates Continued In Vitro Antiviral Activity Against Omicron Subvariants, Including BA.4 and BA.5 – READ, ARCHIVE
July 30, 2022 – Arkmedic Substack: Welcome to Gilead – A scientific scandal with huge implications for women’s health is brewing and you weren’t going to hear about it – until now – Women’s cancer to accelerate – READ
[from the makers of remdesivir, so I’ll put this here] – Another paper RETRACTED! [peer review or peer pressure!]
July 15, 2022 – Vaccine Safety Research Foundation Episode #38: The Tragedy of Remdesivir – WATCH
April 21, 2022 – Gilead Statement on WHO Recommendation of Veklury® (Remdesivir) and Acceleration of Prequalification Submission – READ, ARCHIVE
- “World Health Organization’s (WHO) Therapeutics and COVID-19: living guideline, which now conditionally recommends Veklury® (remdesivir) for use in the treatment of patients with non-severe COVID-19 at the highest risk of hospitalization.” – WHO document – ARCHIVE
- “The revised recommendation is based on evidence from our Phase 3 double-blind, placebo-controlled trial (PINETREE) demonstrating that a three-day course of Veklury treatment significantly reduced the risk of hospitalization for non-hospitalized patients at high risk of disease progression.” [They’re testing remdesivir as EARLY treatment – finally!!! – so like Hydroxychloroquine but remdesivir is highly TOXIC]


February 23, 2022 – Stew Peters: REMDESIVIR HAS BEEN AUTHORIZED FOR USE IN INFANTS – w/ Ali Shultz – infants separated from parents and force remdesivir – WATCH, CREDIT
February 5, 2022 – TGAustralia: TGA approves provisional determination for Gilead Sciences Pty Ltd for COVID-19 treatment, VEKLURY (remdesivir), for proposed use in children and adults who are at risk of progressing to severe COVID-19 – ARCHIVE
February 2, 2022 – The Water Cooler w/ David Brody: Dr. Paul Marik Calls Remdesivir Policy an “Absolute Disgrace” – WATCH, CREDIT
January 21, 2022 – The David Knight Show: HHS Targets Kids, Ends Adult Data Collection – on EUA of Remdesivir for children – WATCH, CREDIT,
- David Knight summarizes the history of Veklury® (remdesivir) and raises the issue of data manipulation regarding the health of children
January 21, 2022 – FDA PRESS RELEASE: FDA Takes Actions to Expand Use of Treatment for Outpatients with Mild-to-Moderate COVID-19 and to pediatric population down to 3.5kg weight! – READ Three days later monoclonal antibodies were removed as an early treatment option!
January 21, 2022 – Gilead PRESS RELEASE: FDA Approves Veklury® (Remdesivir) for the Treatment of Non-Hospitalized Patients at High Risk for COVID-19 Disease Progression – READ
- “Approval Based on Phase 3 Data Showing Veklury Significantly Reduced Risk of Hospitalization By 87% Compared with Placebo”
- NIH Guidelines Recommend Veklury for the Treatment of Non-Hospitalized Patients at High Risk
- FDA Expands Pediatric Emergency Use Authorization (EUA) to Include Treatment of Non-Hospitalized Pediatric Patients at High Risk
January 27, 2022- NEJM – Early Remdesivir to Prevent Progression to Severe COVID-19 in Outpatients (3-day course remdesivir) – READ
2021
November 17, 2021 – Association of American Physicians and Surgeons (AAPS): Biden’s Bounty on Your Life: Hospitals’ Incentive Payments for COVID-19 – READ
- The ‘Coronavirus Aid, Relief, and Economic Security Act’’ or the ‘‘CARES Act’’ March 27, 2020 allowed for these incentives
- “A 20 percent “boost” bonus payment from Medicare on the entire hospital bill for use of remdesivir instead of medicines such as Ivermectin.”
October 18, 2021 – The Blaze: Horowitz: The $cience of remdesivir vs. ivermectin: A tale of two drugs – READ, ARCHIVE
- “A tale of two drugs. One has become the standard of care at an astronomical cost despite studies showing negative efficacy, despite causing severe renal failure and liver damage, and despite zero use outpatient. The other has been safely administered to billions for river blindness and now hundreds of millions for COVID throughout the world and has turned around people at death’s doorstep for pennies on the dollar.”
October 13, 2021 – Cardiovascular Toxicity: Potential cardiotoxic effects of remdesivir on cardiovascular system: a literature review. (Remdesivir is substantially more cardio-toxic than chloroquine) – READ, WATCH
~August 27, 2021 – Awaken America tour: Dr Brian Ardis warns about remdesivir – WATCH, CREDIT
July 8, 2021 – In the US “COVID-19 Treatment Guidelines”, the NIH knows remdesivir is associated with renal toxicity – See Table 2e pg. 153 – HERE, ARCHIVE, WATCH The table is now referred to as Table 2f – HERE
April 22, 2021 – E Clinical Medicine: Efficacy of the TMPRSS2 inhibitor camostat mesilate in patients hospitalized with Covid-19-a double-blind randomized controlled trial – Gunst et al – PDF , FUNDING, ARCHIVE, Danish study – READ,
- In this comostat mesiliate trial “some patients received remdesivir and/or dexamethasone as standard of care”!! – CREDIT
- ““Remdesivir was administered to 96 participants; 64 (47%) in the camostat group and 32 (47%) in the placebo group.”” The study had a total of 208 participants in total of which 46% received remdesivir!
- The study found “that 200 mg t.i.d. camostat mesilate is not an effective treatment for hospitalized patients with Covid-19”
April 17, 2021 – India – COVID-19: Remdesivir price slashed by nearly Rs 2000 post govt intervention – READ
April 2021 – Remdesivir and acute renal failure: a potential safety signal from disproportionality analysis of the WHO safety database. (Remdesivir was found to have a 20-fold higher renal failure rate compared to three comparable drugs: hydroxychloroquine, tocilizumab, and lopinavir/ritonavir) – READ, WATCH
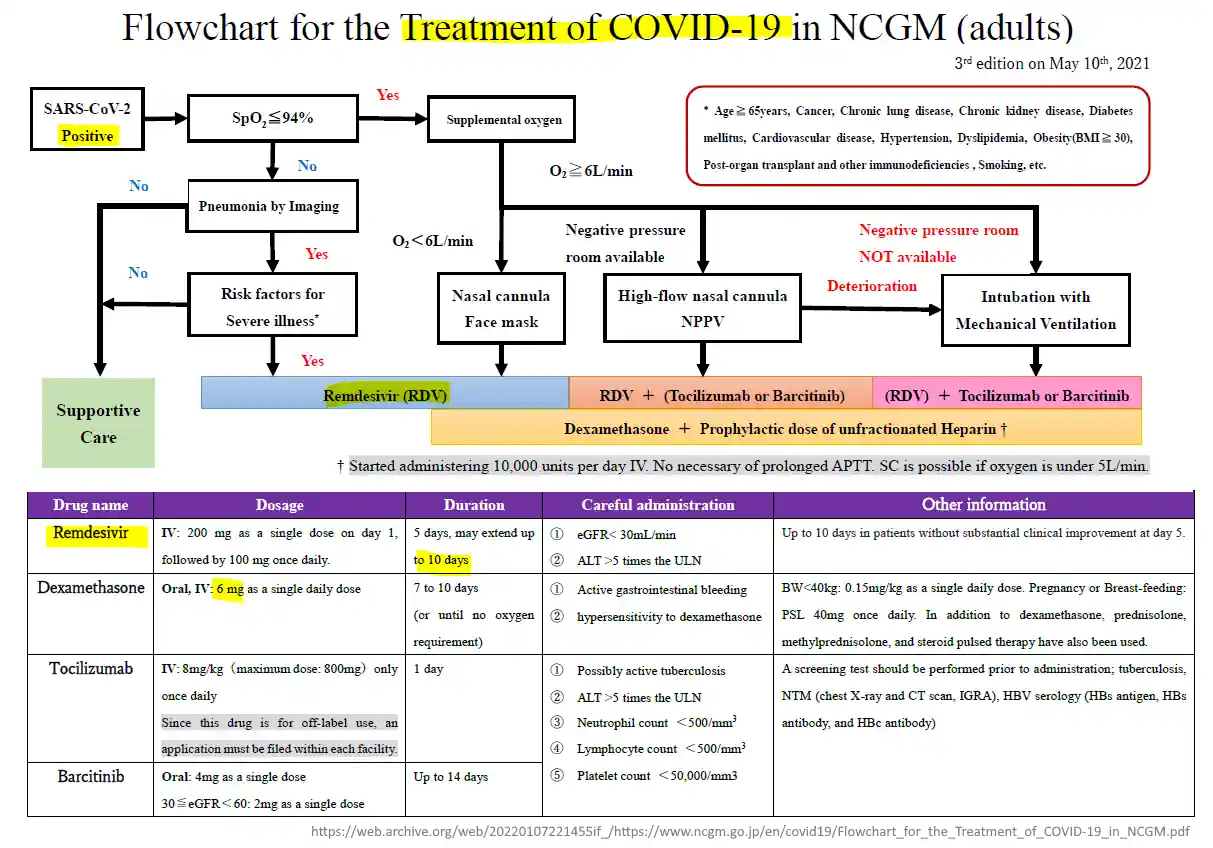
May 10, 2021 – National Center for Global Health and Medicine (NCGM) JAPAN: Flowchart for the Treatment of COVID-19 in NCGM (adults) 3rd Edition – PDF, ARCHIVE, (NCGM date back to 1868! – PDF)
- NCGHM were intimately involved in the management of the Diamond Princess cruise ship and the early trials with Remdesivir.
March 18, 2021 – American Society of Microbiology: Remdesivir for COVID-19: Why Not Dose Higher? – READ, Funded by Steve Kirsh foundation – CREDIT
January 4, 2021 – WCPO: A new study from the University of Cincinnati is raising concerns about the safety of the FDA approved COVID-19 treatment Remdesivir – WATCH, CREDIT
January 2, 2021 – Springer: Remdesivir in Coronavirus Disease 2019 (COVID-19) treatment: a review of evidence by Jaime Lin et al – READ
- “Remdesivir, an investigational broad-spectrum antiviral agent has previously demonstrated in vitro activity against Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2), and in vivo efficacy against other related coronaviruses in animal models.”
2020
November 20, 2020 – NBC News: Remdesivir shouldn’t be used on hospitalized COVID-19 patients, WHO advises (as there is no evidence it improves survival or reduces the need for ventilation). “Remdesivir has potential side-effects on the kidneys, according to data Gilead shared with the European Medicines Agency” – READ, ARCHIVE, READ2
November 19, 2020 – BMJ: WHO Guideline Development Group advises against use of remdesivir for covid-19 – READ
November 5, 2020 – NEJM: Remdesivir for the Treatment of Covid-19 — Final Report by Beigel et al [NIH paper] – READ, CNN – this study was used to gain FDA approval – READ
Dr Alexander discussion re paper – SUBSTACK
- Study design tampering “they made a non patient important outcome (time to recovery), the primary outcome.”
- “the NIH highly touted and flaunted study that did not report or focus on patient-important objective outcomes and only on reduced time to recovery, was deeply flawed methodologically.” – REF
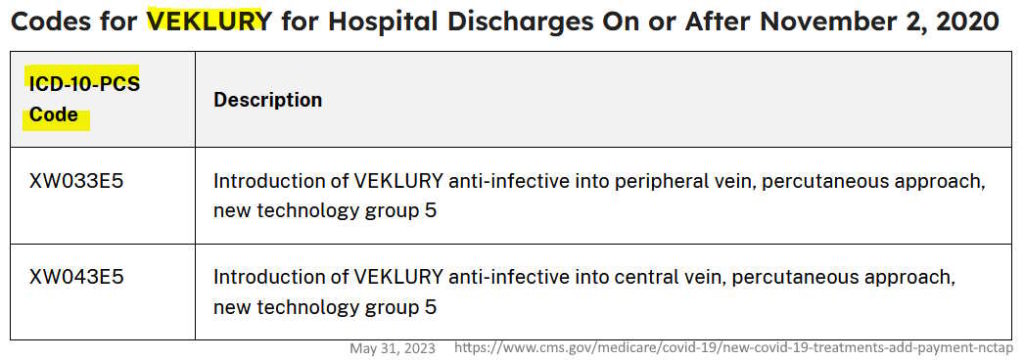
November 4, 2020 – Under it’s New COVID-19 Treatments Add-On Payment (NCTAP) US CMS.gov provides a 20% bonus payment on the entire hospital bill, just if they administer remdesivir. – LINK, LINK2, WATCH Compared to other treatments remdesivir is below inferior (look at cost of the drugs vs those approved!)!
October 29, 2020 – Pulitzer Center: The ‘Very, Very Bad Look’ of Remdesivir, the First FDA-Approved COVID-19 Drug – READ
- On October 15, 2020 “The World Health Organization’s (WHO’s) Solidarity trial showed that remdesivir does not reduce mortality or the time COVID-19 patients take to recover.”
- Science has learned that both FDA’s decision and the EU deal came about under unusual circumstances that gave the company important advantages.” The FDA did not consult their Antimicrobial Drugs Advisory Committee (ADAC).
- The European Union settle on the remdesivir pricing exactly 1 week before the disappointing Solidarity trial results came out – a $1 billion deal!
October 28, 2020 – Science Innsider: The ‘very, very bad look’ of remdesivir, the first FDA-approved COVID-19 drug – The Food and Drug Administration held no advisory meeting on antiviral, and the European Union signed contract without knowing of failed trial – READ
- Gilead Sciences, On 8 October, 2020 Gilead Sciences signed an agreement to supply the European Union with its drug remdesivir as a treatment for COVID-19—a deal potentially worth more than $1 billion. Two weeks later, on 22 October, the U.S. FDA approved remdesivir for hopitalised COVID-19 patients in the United States—the first drug to receive that status.
October 23, 2020 – NBC News: FDA approves first drug for COVID-19: remdesivir. No other Covid-19 drugs have received FDA approval. – READ
October 22, 2020 – The Highwire Ep 186: FAUCI’S REMDESIVIR ‘FALLS FLAT’ – WATCH, BITCHUTE, FULL
- “In April, Dr. Fauci said the repurposed antiviral, Remdesivir, showed a “clear-cut, significant, positive effect in diminishing the time to recovery.” However, the WHO has now released the findings of its ‘Solidarity Trial,’ encompassing 400 hospitals around the world. Results indicate the drug has ‘fallen flat,’ prompting the WHO to declare it has “little to no effect” on hospitalized COVID-19 patients.”
- “This is just a drug looking for a purpose” says Del
October 22, 2020 – CNN: Remdesivir becomes first Covid-19 treatment to receive FDA approval – READ
October 22, 2020 – FDA’s approval of Veklury (remdesivir) for the treatment of COVID-19—the science of safety and effectiveness – READ, REF, Gilead letter – PDF, Product label – PDF
- Approved for adult patients and children (12 years of age and older and weighing at least 40 kg) requiring hospitalization – “the first drug approved to treat COVID-19“
- “FDA also revised the EUA for Veklury, originally issued on May 1, 2020, to permit the drug’s use for treatment of suspected or laboratory confirmed COVID-19 in hospitalized pediatric patients weighing 3.5 kg to less than 40 kg or hospitalized pediatric patients less than 12 years of age weighing at least 3.5 kg.”
- “Gilead submitted a study published in the New England Journal of Medicine that showed the drug shortened the course of illness from an average of 15 days to about 11 days in hospitalized patients.” – REF, Study not peer reviewd until Nov 2020 – STUDY – Time to recovery a poor primary endpoint – SUBSTACK
October 20, 2020 – Reuters: EU makes 1 billion-euro bet on Gilead’s COVID drug before trial results – The European Union agreed to pay > 1 billion euros (USD$1.2 billion) to Gilead for a six-month supply of its antiviral drug remdesivir, before the publication of final results of the biggest trial of the COVID-19 medication “The agency [WHO] is investigating remdesivir’s potential adverse effects on kidneys”. – READ
October 16, 2020 – Science: Remdesivir and interferon fall flat in WHO’s megastudy of COVID-19 treatments – READ, ALT
October 15, 2020 – PRESS RELEASE: Gilead Sciences Statement on the Solidarity Trial – READ, ARCHIVE
- “We are concerned that the data from this open-label global trial have not undergone the rigorous review required to allow for constructive scientific discussion, particularly given the limitations of the trial design.”
October 15, 2020 – NEJM: Repurposed antiviral drugs for COVID-19 –interim WHO SOLIDARITY trial results – READ, preprint date – READ2, WHO SOLIDARITY TRIAL COVID-19 core protocol – PDF
- WHO funded SOLIDARITY trial included: “405 hospitals in 30 countries, 11,330 adults underwent randomization; 2750 were assigned to receive remdesivir, 954 to hydroxychloroquine, 1411 to lopinavir (without interferon), 2063 to interferon (including 651 to interferon plus lopinavir), and 4088 to no trial drug.”
- ALL had “little or no effect on hospitalized patients with Covid-19, as indicated by overall mortality, initiation of ventilation, and duration of hospital stay.”
- The regimen for remdesivir (intravenous) was 200 mg on day 0 and 100 mg on days 1 through 9.
- [note: hydroxychloroquine was not being used in patients this late by the frontline doctors – MORE]
October 15, 2020 – HHS | Public Health Emergency: Veklury (remdesivir): ASPR’s Portfolio of COVID-19 Medical Countermeasures under Investigation – ARCHIVE, SOURCE, READ, Includes timeline, FAQ and distribution linkes for Veklury!
- “October 1, 2020: Updated EUA released; USG oversight of the allocation and distribution of Veklury (remdesivir) is no longer required as supply is greater than demand by U.S. hospitals….”
- About the Agreement with Gilead Science, Inc. and AmerisourceBergen (the sole distributor for remdesivir) – ARCHIVE [FYI these drug wholesaler merged in 2001 – REF]
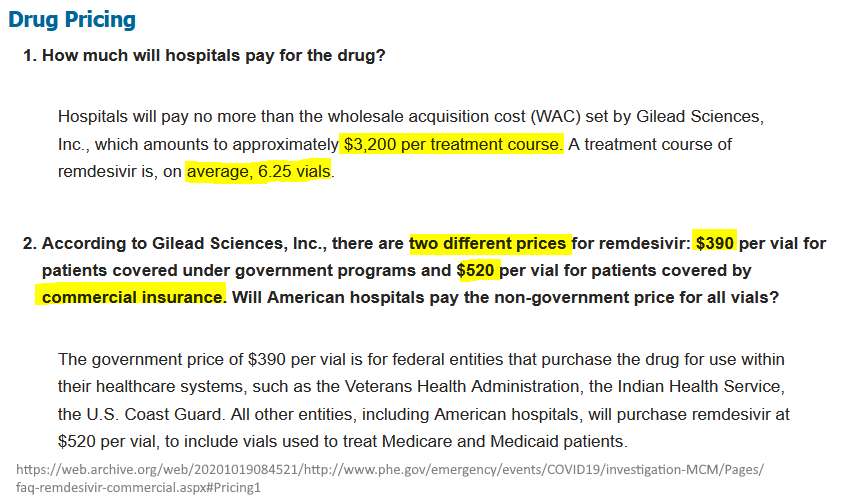
Drug Pricing – ARCHIVE
October 3, 2020 – The Lancet correspondence: Remdesivir and COVID-19 – READ
September 16, 2020 – Infectious Diseases Society of America (IDSA) | COVID-19 Real-Time Learning Network – curated review of key information and literature about Remdesivir [cites research studies]- ARCHIVE
- IDSA funded by CDC grant number 6 NU50CK000477-04-01
- August 21, 2020 – Effect of Remdesivir vs Standard Care on Clinical Status at 11 Days in Patients With Moderate COVID-19: A Randomized Clinical Trial – Spinner et al – READ
- May 22, 2020 – NEJM (Preprint): Remdesivir for the Treatment of Covid-19 — Final Report by Beigel et al ACTT-1 Study Group Members – READ (Adaptive COVID-19 Treatment Trial (ACTT): An NIH-sponsored randomized, double-blind, placebo-controlled adaptive trial – TRIAL)
- Trial protocol CHANGED mid trial! Originally had “death” on thier “7-point ordinal scale ” for primary outcome measure (Feb 21, 2020) – ARCHIVE, by April 27, 2020 they changed the trial design to be only “Time to recovery” as primary outcome measure – ARCHIVE [See Feb 21, 2020 below for screenshots]
- May 27, 2020 – SIMPLE-Severe Trial: Gilead-sponsored multinational, open-label trial of remdesivir in patients with severe COVID-19 – NEJM: Remdesivir for 5 or 10 Days in Patients with Severe Covid-19 by Goldman et al – READ, Clinical Trial protocol (Dec 31, 2019) – READ
- April 29, 2020 – The Lancet: Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial – Wang et al – READ, ARCHIVE – In China “Due to under-enrollment, the trial was stopped early and was likely under-powered.”
- Other Remdesivir clinical trials – SEARCH, ARCHIVE
September 11, 2020 – HHS | Office of the Assistant Secretary for Preparedness and Response (ASPR): Update and Guidance on U.S . Government Allocation and Distribution of Remdesivir – Unclassified – SLIDES, ARCHIVE
- US govt received 940,000 “vials” DONATED by Gilead, HHS purchases 500,000 “treatment courses” from July-Sept 2020 – at 6.25 vials/ave course = 3,125,000 vials+ – SLIDE 6, As of Sept 10, 2020 the HHS has allocated 76,644 cases (3,065,760 vials or 490,522 treatment courses) -see Slide 10
- Hospitals report data into “HHS Protect” (SLIDE 11) – “HHS Protect is a secure decision-making and operations platform for the whole-of-government response to the COVID-19 pandemic.” – PDF
August 28, 2020 – PRESS RELEASE: Gilead’s Investigational Antiviral Veklury® (Remdesivir) Receives U.S. Food and Drug Administration Emergency Use Authorization for the Treatment of Patients With Moderate COVID-19 – Expands Previous Authorization of Veklury to Treat Hospitalized Patients with COVID-19 Regardless of Oxygen Status – READ
August 13, 2020- Clin. Epid. & Global Heath: Remdesivir and its antiviral activity against COVID-19: A systematic review – READ
July 10, 2020 – Nature Medicine: Extrapulmonary manifestations of COVID-19 – READ, WATCH
- These are all side effects of remdesivir, yet the study concludes they are attributed to the virus SARS-CoV-2:
“Although COVID-19 is most well known for causing substantial respiratory pathology, it can also result in several extrapulmonary manifestations. These conditions include thrombotic complications, myocardial dysfunction and arrhythmia, acute coronary syndromes, acute kidney injury, gastrointestinal symptoms, hepatocellular injury, hyperglycemia and ketosis, neurologic illnesses, ocular symptoms, and dermatologic complications.”
June 30, 2020 – Case report study of the first five COVID-19 patients treated with remdesivir in France (the remdesivir treatment was interrupted in 4 out of 5 patients) – READ, WATCH
June 28, 2020 – HHS signed a memorandum of agreement with Gilead Sciences, Inc – READ, SOURCE
June 11, 2020 – NEJM – Compassionate Use of Remdesivir for Patients with Severe COVID-19 (study by Gilead 10 days of drug of which 60% patients reported adverse events including acute kidney failure) – READ, WATCH
May 28, 2020 – The Lancet – Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial by Wang et al – READ, PDF, (note this was in preprint April 29, 2020), Dr Alexander – SUBSTACK
- “In adult patients admitted to hospital for severe COVID-19, “remdesivir was not associated with statistically significant clinical benefits.”
- Furthermore, and very alarmingly, adverse events were reported in “102 (66%) of 155 remdesivir recipients versus 50 (64%) of 78 placebo recipients.” –REF
May 27, 2020 – NEJM: Remdesivir for 5 or 10 Days in Patients with Severe Covid-19 by Goldman et al – READ
- SIMPLE-Severe Trial: Gilead-sponsored multinational, open-label trial of remdesivir in patients with severe COVID-19 – SOURCE
May 26, 2020 – MedRxiv Preprint: Remdesivir use in patients with coronavirus COVID-19 disease: a systematic review and meta-analysis of the Chinese Lancet trial with the NIH trial – by Paul Alexander et al – READ, CREDIT
- Dr Alexander “when working for the World Health Organization (WHO) and the Pan American Health Organiization in Washington DC, published a paper in May 2020 that served to promote the use of Remdesivir. It was simply a review of 2 clinical trials that claimed a reduction in time to recovery and reduced risk of Serious Adverse Events” – REF
May 14, 2020 – Bloomberg: All eyes on Gilead – READ, ARCHIVE, Wikipedia – CREDIT, Comments from “Ken Kent, Gilead’s vice president in charge of chemical development and manufacturing…”
- “In mid-January [2020], Kent got a call from Reza Oliyai, senior vice president for Gilead’s pharmaceutical operations, telling him the company would need to make large quantities of remdesivir to fight the novel coronavirus.”
- “Remdesivir is tricky to produce—the monthslong process involves 70 raw materials, reagents, and catalysts”… and approximately “25 chemical steps in the production process. Most drugs require about half that number. Some of the steps are more delicate than others. An early one uses a reagent so flammable it will spontaneously combust if exposed to air. Another involves a poison called trimethylsilyl cyanide.” – REF
- Symptoms of cyanide poisoning includes kidney failure, the same symptom remdesivir recipients often experience!
- “The original end-to-end manufacturing process required 9 to 12 months to go from raw materials at contract manufacturers to finished product, but after restarting production in January 2020, Gilead Sciences was able to find ways to reduce the production time to six months” – REF
May 7, 2020 – Japan approves Gilead Sciences’ remdesivir as COVID-19 drug – READ
May 7, 2020 – PRESS RELEASE: Gilead Announces Approval of Veklury® (remdesivir) in Japan for Patients With Severe COVID-19 – READ, ARCHIVE
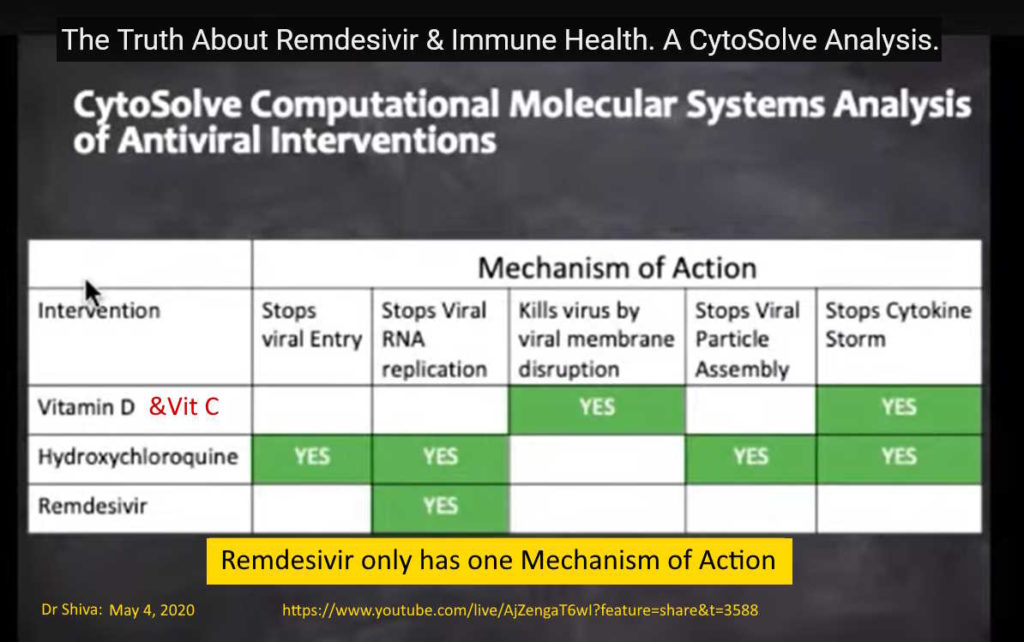
May 4, 2020 – Dr Shiva Ayyadurai: Dr.SHIVA LIVE: The Truth About Remdesivir & Immune Health. A CytoSolve Analysis – presentation on viruses, the immune system and remdesivir – WATCH, What is Cytosolve? – EXCERPT, FULL, BACKUP –
- Remdesivir triphosphate (RDV-TP) (Gilead # GS-441524) inhibits the viral enzyme RNA-dependent RNA Polymerase (RDRP) – it mimics Adenine. RDRP is necessary for viral RNA replication in cells
- Gilead trial discussed – remdesivir – 71% (5 day tmt arm) and 74% (10 day tmt arm) people had ADVERSE EFFECTS!, [no placebo group], which included Acute Respiratory Failure & Liver dysfunction! – EXCERPT
May 1, 2020: FDA issued an Emergency Use Authorization (EUA) for the emergency use of Veklury® (remdesivir) for treatment of hospitalized patients with severe 2019 coronavirus disease (COVID-19) – PDF, ARCHIVES, Remdesivir – FDA
- On January 21, 2022 – the EUA was REVOKED
April 30, 2020 – The Highwire Ep 161: CORONAVIRUS: A NATION DIVIDED – segment on Chloroquine vs. Remdesivir – WATCH, FULL
April 29, 2020 – NY Post: Dr. Fauci praises remdesivir after breakthrough in coronavirus treatment – WATCH & READ, In the Whitehouse – EXCERPT
- “reduced coronavirus recovery time” – [the trial was not complete and the endpoint were changed after the trial was set]
April 29, 2020 – NY Post: Gilead says remdesivir shows ‘positive’ signs for coronavirus treatment – READ
- The firm teased an upcoming briefing by NIAID [Dr Fauci] that would offer “detailed information” about the results.
- “The company also announced its own study showed that 62% of patients treated early with remdesivir were discharged from the hospital, compared with 49% of patients who were treated late.”
April 29, 2020 – LA Times: Clinical trial of remdesivir may be a turning point in coronavirus fight – READ
- Government researches suggest antiviral medication remdesivir helped patients with advanced COVID-19 recover 31% faster than a placebo treatment. – 1,063 patients enrolled in trial
- “The early results, emerging from a large clinical trial sponsored by the National Institute of Allergy and Infectious Diseases, appears to position the drug as the standard therapy for hospitalized COVID-19 patients going forward.”
- “the benefits were so clear that researchers halted this portion of the trial early. As they move on to investigate other drugs against COVID-19, researchers will give all trial participants remdesivir and will make the antiviral the new standard against which other drugs are compared, Fauci said.”
- [Dr Marik comments the trial was fraudulent – WATCH]
- China study with remdesivir found “no significant difference in how sick patients became or how quickly they recovered” – The Lancet – STUDY
“What [the clinical trial] has proven is that a drug can block this virus,”…“This drug happens to be blocking an enzyme that the virus uses.”
Fauci said on Wednesday in the Oval Office of the White House
April 29, 2020 – Gilead PRESS RELEASE – Gilead Sciences Statement on Positive Data Emerging From National Institute of Allergy and Infectious Diseases’ Study of Investigational Antiviral Remdesivir for COVID-19 – READ, ARCHIVE
April 29, 2020 – Gilead PRESS RELEASE – Gilead Announces Results From Phase 3 Trial of Investigational Antiviral Remdesivir in Patients With Severe COVID-19 – Study Demonstrates Similar Efficacy with 5- and 10-Day Dosing Durations of Remdesivir – READ, ARCHIVE
April 29, 2020 – The Lancet: Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial – Wang et al (CHINA) – READ, LA Times – ARTICLE
- Hospitalised patients – 158 received remdesivir (GS-5734) and 79 placebo
- the found “no significant difference in how sick patients became or how quickly they recovered” – ARTICLE
[Yet the same day this was published Fauci announced remdesivir as the standard of care for COVID-19 in hospitals in the US] - “Adverse events were reported in 102 (66%) of 155 remdesivir recipients versus 50 (64%) of 78 placebo recipients. Remdesivir was stopped early because of adverse events in 18 (12%) patients versus four (5%) patients who stopped placebo early.”
- [Note LA Times claimed remdesivir was “used effectively against Ebola” this is untrue, it was pulled from the ebola trials, it was increasing mortality!- ARTICLE]
April 26, 2020 – COVID-19 Early Treatment Fund: Entrepreneur and philanthropist Steve Kirsch founded CETF to fund outpatient clinical trials of repurposed drugs – Website first ARCHIVED, READ,
- “This site describes the fastest way to defeat the virus.” Early treatment FAQ – READ
April 24, 2020 – CNBC: Coronavirus: Remdesivir had no effect on patients, according to leaked Gilead study on using Remdesivir to treat Covid-19 – Hannah Kuckler, Financial Times – WATCH, CREDIT
- WHO allegedly prematurely published results from the first RCT on Remdesivir conducted in China where 237 patients were enrolled of which 158 were given remdesivir (the study planned to enrol 453 but fell short!). Mortality at 28 days was 13.9% drug, 12.8% placebo control
- But Gilead believe the WHO’s post had ‘inappropriate characterization of the study’
- Note on Feb 2, 2020 – 50 Tons of Vitamin C was trucked into Wuhan for healthcare workers etc, and even IV vit C trials couldn’t enrol enough patients – HERE
April 19, 2020 – In Jan & Feb 2020 remdesivir trials for COVID-19 were conducted, on April 19, 2020 the last patient was enrolled in the NIH trial according to Dr Marik. Ten days later (April 29, 2020), before the study had terminated, Dr Fauci announced in the oval office the trial was “good news”! – WATCH
April 19, 2020 – Microbiology Notes: Remdesivir- Mechanism of Action, Uses, Synthesis & COVID-19 – ARCHIVE, Dr Shiva – CREDIT, Mode of Action – IMAGE
April 17, 2020 – NIH: Antiviral remdesivir prevents disease progression in monkeys with COVID-19 – Study supports clinical testing under way across U.S. – READ, CREDIT
April 15, 2020 – PRESS RELEASE: Gilead Provides Additional Information Regarding 2020 Annual Meeting of Stockholders Due to COVID-19 Precautions – READ, ARCHIVE
April 11, 2020 – Infectious Disease Society: Infectious Diseases Society of America Guidelines on the Treatment and Management of Patients with COVID-19 – ARCHIVE
- “The guideline panel suggests against remdesivir for routine treatment of patients with oxygen saturation >94% and no supplemental oxygen; however, strongly urges continued study through recruitment into RCTs”
- The mode of action of remdesivir is only “acts by causing premature termination of viral RNA transcription” – [is viral replication even an issue in this stage of disease progression in hospitalised patients? Especially given the drugs “toxicity profile”.]
- “Patients receiving five days of remdesivir may experience fewer SAEs and adverse events leading to treatment discontinuation than patients receiving 10 days of remdesivir…”

April 10, 2020 – NEJM: Compassionate Use of Remdesivir for Patients with Severe Covid-19 – Grein et al (Gilead Sciences) – PDF
April 8, 2020 – PRESS RELEASE: Gilead Announces $20 Million Philanthropic Fund to Support Nonprofit Organizations Impacted by the COVID-19 Crisis – READ, ARCHIVE
- “Gilead Sciences, Inc. (Nasdaq: GILD) announced the creation of the global Gilead CARES (COVID-19 Acute Relief and Emergency Support) Grantee Fund to provide financial support to current grantees facing an imminent closure or termination of vital services due to losses attributable to the COVID-19 pandemic. The fund will provide up to $20 million in donations to these nonprofit groups.”
March 25, 2020 – National Center for Global Health & Medicine (Japan): NCGM Fights against COVID-19 – Lessons Learned (2nd Edition) – PDF, CREDIT, NCGM – HERE, ARCHIVE
- Reported investigator-initiated clinical trials were launched using Remdesivir, as part of “multi-regional clinical trials in collaboration with the NIH (National Institutes of Health, USA)”.
March 5, 2020 – NEJM: First Case of 2019 Novel Coronavirus in the United States – Holshue et al – READ
February 28, 2020 – Scientific America: A Promising Antiviral Is Being Tested for the Coronavirus—but Results Are Not Yet Out – READ, ARCHIVE
- The drug remdesivir is effective against many other viruses, and some experts are optimistic that it—or similar compounds—may work for the pathogen responsible for COVID-19
- …”and a malaria drug called chloroquine, which is not an antiviral but has shown some efficacy against COVID-19 in a lab dish. Yet experts say drugs that specifically target other pathogens are unlikely to work well enough.”
- “Timothy Patrick Sheahan, an assistant professor of epidemiology at the University of North Carolina Gillings School of Global Public Health, is among those in the U.S. working on antiviral drugs for COVID-19. Like De Clercq, he is skeptical that many of the antivirals already on the market would work.
“I’m doubtful that existing approved medications for other infectious diseases will have some magical property against this new coronavirus,”
Tim Sheahan
- [Note Sheahan has a conflict of interest in making this statement as he helped develop remdesivir otherwise known as GS-5734 ]
- “Sheahan and his colleagues have published several papers showing that remdesivir is effective against SARS, MERS and related bat coronaviruses, as well as some of the common cold coronaviruses.” – HERE & HERE
- “Lisa Gralinski, an assistant professor of epidemiology and colleague of Sheahan’s at the Gillings School,
- “I think it will probably be really effective” if you can get it to the patient within the first or second week… But “you’re not going to be able to come in and give this drug to someone who’s approaching end-stage lung disease and improve their outcome.” At that point, the lung damage is no longer being caused by viral replication but is happening because of the body’s own immune response—so an antiviral would likely not be effective. Yet if enough of the drug is available, Gralinski says, she would give it at the time of diagnosis.”
- “…if we already have something that’s mostly through development, like has luckily been the case with remdesivir, you can get it to people very rapidly.” Even if the drug proves to be effective, however, producing enough of it and distributing it to everyone in need is not guaranteed.”
February 26, 2020 – Senator Rand Paul’s wife Kelley buys stocks in Gilead – SOURCE
February 26, 2020 – PRESS RELEASE: Gilead Sciences Initiates Two Phase 3 Studies of Investigational Antiviral Remdesivir for the Treatment of COVID-19 – READ, ARCHIVE
- “Gilead’s trials will evaluate two dosing durations of the drug, which is given intravenously. The randomized, open-label, multicenter studies will enroll about 1,000 patients mostly in Asia, as well as in countries that have had high numbers of diagnosed cases. The trials are planned to start in March.
- These trials are on top of two clinical trials in China’s Hubei province led by the China-Japan Friendship Hospital and a recently launched trial in the U.S. led by the NIAID. Gilead donated the drug and provided scientific expertise for those trials. The China trial data is expected in April.” – REF
February 25, 2020 – NIAID: NIH Clinical Trial of Remdesivir to Treat COVID-19 Begins – Study Enrolling Hospitalized Adults with COVID-19 in Nebraska – READ, ARCHIVE
- Clinical Trial begun at the University of Nebraska Medical Center (UNMC) in Omaha – sponsored by NIAID – Clinical Trial NCT04280705 – HERE
- “UNMC’s National Quarantine Unit is supported by the office of the Assistant Secretary for Preparedness and Response (ASPR) at the Department of Health and Human Services. It has a 20-bed capacity and is in close proximity to the Nebraska Biocontainment Unit, should a higher level of care be needed. Clinical trial participants are cared for in the biocontainment unit.”
February 21, 2020 – ClinicalTrials.gov: Adaptive COVID-19 Treatment Trial – READ, ARCHIVE, That trial will be conducted at up to 50 sites around the world and will test remdesivir against a placebo. – SOURCE
- April 27, 2020 – NIAID changed clinical trial trial Primary Outcome Measure, to time to recovery and removing “death” from the criteria – ARCHIVE


February 18, 2020 – Gilead Sciences Update On The Company’s Ongoing Response To COVID-19 – ARCHIVE, LIVE, All ARCHIVES
- “Remdesivir is an investigational nucleotide analog with broad-spectrum antiviral activity – it is not approved anywhere globally for any use.”
- “This is an experimental medicine that has only been used in a small number of patients with COVID-19 to date, so Gilead does not have an appropriately robust understanding of the effect of this drug to warrant broad use at this time.”
- “Remdesivir has demonstrated in vitro and in vivo activity in animal models against the viral pathogens MERS and SARS, which are also coronaviruses and are structurally similar to COVID-19.” [so to did cheap Hydroxychloroquine, with a history and safety profile]
- To date “two studies are being coordinated by the China-Japan Friendship Hospital and are being conducted at multiple sites in Hubei province” China.
- The study in patients with severe disease began enrolling patients on February 6, 2020 – TRIAL
- The study in patients with moderate disease began enrolling patients on February 13, 2020
February 6, 2020 – ClinicalTrails. gov: Severe 2019-nCoV Remdesivir RCT – READ, ARCHIVE, SOURCE
February 6, 2020 – New York Times: China Begins Testing an Antiviral Drug [remdesivir] in Coronavirus Patients – READ
February 5, 2020 – ClinicalTrails. gov: Mild/Moderate 2019-nCoV Remdesivir RCT – READ, ARCHIVE
February 5, 2020 – Washington Times: Chinese scientists ask for patent on U.S. drug to fight virus – READ
Gilead, headquartered in Foster City, California, said it applied in 2016 for a Chinese patent on use of remdesivir against coronaviruses and is waiting for a decision.
January 31, 2020 – Gilead Sciences Statement on the Company’s Ongoing Response to the 2019 Novel Coronavirus (2019-nCoV) – READ
January 21, 2020 – The antiviral Remdesivir was used on the first COVID-19 patient in the US, given on the evening of day 7 of hospitalisation – TIMELINE, Daoyu15 Substack: Study of the USA-WA-1 case report… – READ, Also – READ
- But the patient had cleared the virus by day 3 of hospitalisation (as indicated by higher PCR cycles needed indicating viral titers dropped) and when pneumonia began to develop on the 6th day of hospitalization, was subjected to antibiotics treatment – REF
- Remdesivir given evening of hospitalisation day 7 at the same time the antibiotic Vancomycin was stopped, the next day the patient “clinical conditions improved”. Remdesivir was used after the virus had cleared! and used in conjunction with other treatments, yet this case study was justification for clinical trials!
January 2020 – “In mid-January, [Ken] Kent got a call from Reza Oliyai, senior vice president for Gilead’s pharmaceutical operations, telling him the company would need to make large quantities of remdesivir to fight the novel coronavirus.” – REF
2019
December 31, 2019 – Clinical Trials.gov | Gilead Sciences-sponsored : Study to Evaluate the Safety and Antiviral Activity of Remdesivir (GS-5734™) in Participants With Severe Coronavirus Disease (COVID-19) – READ, ARCHIVE March 3, 2020 (Phase 3 Not yet recruiting) – ARCHIVE, Protocol – PDF, History of initial study changes – ARCHIVE
- May 27, 2020 – SIMPLE-Severe Trial: Gilead-sponsored multinational, open-label trial of remdesivir in patients with severe COVID-19 – NEJM: Remdesivir for 5 or 10 Days in Patients with Severe Covid-19 by Goldman et al – READ, SOURCE
December 24, 2019 – [Bloomberg: All eyes on Gilead – REF] – Tomas Cihlar, VP for discovery virology at Gilead Sciences Inc., received a email from a top infectious diseases expert at the University of Virginia.
- “There were cases of pneumonia suddenly emerging in Wuhan, China. Watch this one, the virologist warned. It might be a new coronavirus.”
- “Remdesivir was one of the few experimental medicines that had shown promise in lab studies against a wide variety of coronaviruses.” [i.e. in vitro like Chloroquine in 2005]
- Gilead started planning “on the assumption” the new virus would turn into a pandemic…”Within weeks, Chief Executive Officer Daniel O’Day formed a task force to study how to test remdesivir and, if it worked, mass-produce it”
December 12, 2019 – NEJM: A Randomized, Controlled Trial of Ebola Virus Disease Therapeutics – (monoclonal antibodies vs remdesivir), funded by NIAID. [The study showed 28 days of remdesivir increased mortality of 53.1% and pulled from the trial] – READ, PDF, WATCH, Dr Marik comments – WATCH,
- Remdesivir dropped from Ebola trial in DRC – increased risk of death & kidney failure- TIMELINE
November 27, 2019 – NIAID: Investigational Drugs Reduce Risk of Death from Ebola Virus Disease
Study Leaders Publish Results from NIH-DRC-WHO Clinical Trial of Four Experimental Therapies – READ
September 2019 – Antiviral Research: Broad spectrum antiviral remdesivir inhibits human endemic and zoonotic deltacoronaviruses with a highly divergent RNA dependent RNA polymerase – Brown with Baric & Sheahan et al – READ, SOURCE
August 13, 2019 – Clinical Trials Arena: Preliminary findings from the Pamoja Tulinde Maisha (PALM) clinical trial have revealed that two monoclonal antibodies, REGN-EB3 and mAb114, produced a higher chance of survival in Ebola patients. (not remdesivir) – READ
“Reuters noted that around 29% of subjects treated with REGN-EB3 and 34% in the mAb-114 arm died, compared to 49% of those on ZMapp and 53% who received remdesivir.”
August 12, 2019 – Science: Finally, some good news about Ebola: Two new treatments dramatically lower the death rate in a trial (not remdesivir!) – READ
August 9, 2019 – Remdesivir dropped from Ebola trial – TIMELINE
2018
March 6, 2018 – American Society of Microbiology: Coronavirus Susceptibility to the Antiviral Remdesivir (GS-5734) Is Mediated by the Viral Polymerase and the Proofreading Exoribonuclease – Agostini, with Baric & Sheahan et al – READ, SOURCE
2017
August 31, 2017 – Gillings School researchers receive $6M+ grant to fight infectious diseases – (Ralph Baric) – READ
History of Gilead Sciences – LINK