The “vaccine only” solution to get the world out of the pandemic had, in 2020, a not-so-obvious agenda which was to establish a new and novel technology product, mRNA gene therapy experimental concept, as the next generation vaccine, a new vaccine platform.
A platform is the back bone technology that can be used over and over to create any new “vaccine” product, to be coded for any disease pathogen. The pandemic simply served as a justification to test the “proof of concept” and normalise the novel, experimental product the eyes of the public – calling it a vaccine helped, as the public were already conditioned to believe that “vaccines” are “safe and effective” – hand in glove kind of relationship!
In March 2019, in Moderna’s SEC filing, they acknowledged the FDA considered mRNA a “gene therapy”. But by early 2020 their mRNA product was now classed as a “vaccine” – simply because it coded for a viral protein. The FDA and other global regulators seemed to simply accept the product as a “vaccine” and treated it as such! Vaccines go through different regulatory pathways than drugs or likely gene therapy products!
Speed is the key justifier for mRNA as a “vaccine”
The key driver for the justification of using the mRNA “vaccine” technology is the speed with which a new product can be developed, produced and delivered – in as little as 60 days from detection of an outbreak!
On the other hand “traditional vaccine development… is very time-consuming and cannot respond instantaneously against novel pandemics such as COVID-19.” [1]
The reason speed is desired is the public health officials believe the threat of “emerging disease” is increasing as a result of population growth and climate change!
Add to this, now that the PCR test have been publicly accepted as a tool to diagnose any disease, whether symptoms are present or not, these new mRNA product become a veritable money tree for big pharma, the network of public-private science grantees and philanthropists like Bill Gates!
All they need is the WHO to get their Pandemic Treaty passed [let’s not!] and they have the power to declared a public health emergency of international concern (PHEIC) for just about anything, pump out an mRNA “vaccine” in 60 days, and mandate the shot to everyone (because they got away with it before!), or you can’t move about in society if you don’t…because by then the programmable digital currency will be in place…but I digress.
Vaccine platform vs vaccine product
A vaccine product targets a particular virus (or variant of) which is said to cause a disease. In the case of COVID-19, the mRNA coded for a particular region (the spike protein) of a particular strain of SARS-CoV-2 virus genetic code. The vaccine does not have any virus in the vial, but rather the blueprint for which to turn the human body into a foreign protein manufacturing site!
A vaccine platform is the technology behind the making of the resultant targeted vaccine product.
In the case of the mRNA vaccine platform, the consistent features are the lipid nanoparticles which encapsulate any “target” synthetic, mNA code. To evade breakdown by the immune system, the code is modified to contain pseudouridine, how long this remains in the body is unknown, it does not act like natural mRNA.
“[t]o build an mRNA vaccine, scientists only need access to the genetic sequence of SARS-CoV-2, and not the actual virus“.
Pfizer, April 2020
In this mRNA vaccine platform, it is the genetic code which can be changed at will – the RNA which encodes for whatever foreign protein the maker desires. We watched this happen with the introduction of the bivalent booster vaccines – by combining the original, but extinct Wuhan spike code with a new variant, an Omicron BA.1 mRNA spike code, and then shortly after BA.4 plus BA.5 mRNA spike codes. The logic for keeping the original spike code is likely to keep some remnant of the product that was used in the emergency clinical trials, as little or no trials were conducted with the new variant codes.
It seems to simply be assumed [or outrightly ignored] by the regulators that foreign proteins will be “safe” no matter what quantity the body is forced to manufacture by the injected technology! Science has already revealed that the SARS-CoV-2 spike proteins are toxic – causing all manner of adverse events – and that’s just the start.
By mid-April 2023 the FDA no longer authorised the original Wuhan “monovalent” spike vaccine, it was simply replaced with the bivalent product. This sets up a precedent for the mRNA vaccine platform.
Mass vaccination – “proof of concept” for next generation vaccines
The mass vaccination campaign was a proof-of-concept for fast-tracked vaccine products, to show the world how useful and “life saving” the new and novel gene therapy mRNA platform could perform. By calling the gene therapy a “vaccine”, as it encodes for an infectious disease virus protein, allowed the novel, never-used-before product to by-passed the more rigorous testing required for drug approval. The regulatory standards for “Biologics” are less stringent – even before the emergency use priorities were applied. The FDA admits vaccine products don’t even need to prevent infection or stop transmission!
The next generation vaccine platform had now been accepted in the eyes of much of the public. So expect the regulators to start approving mRNA products left right and centre – the trials were those done for COVID-19 – and they are highly questionable.
Upscaling manufacturing
The “vaccine” products used in the COVID-19 clinical trials were made on a small scale. Manufacturing was upscaled for the products that were injected into the arms of the masses. We know that greater adverse events and death were associated with certain batch numbers, indicating that production, the final product in the vial, and/or the mixing of the vials were not consistent – and had dire health and life consequences!
We also know that there is DNA contamination in the vials greater than acceptable limits, which could also have devastating health consequences to the recipients.
The lipid nanoparticle ingredients skipped safety tests – not to mention they travel everywhere around the body, and slip the mRNA into any cell – which the body then attacks!
There are so many alarm bells with the vaccine product, that for regulators to be able to justify full approval or registration of these products is alarming….let alone using that same regulatory submission to justify approving the underlying vaccine platform for any future vaccine product.
But governments around the world started building manufacturing plants before these product were even fully approved! – Australia, UK, Canada & Kenya
Once the vaccine platform is approved, no more safety assessment will be required….as if it was done adequately in the first place.
The regulators failed to do their job!
Regulators claim that vaccines go through “rigorous scientific and regulatory processes”, but the Pfizer documents reveal a different story.
The regulators hid or ignored so much death and destruction along the way, which started with the actual clinical trial science! But worse, they don’t pull these products they keep going – although Switzerland just pulled all their COVID-19 vaccines, and Denmark have ended the jab for under 50’s.
Fast tracking the mRNA platform was a goal before 2020
Approving the new technology vaccine platforms, especially an mRNA vaccine platform, appears to have been a goal well before 2020:
- October 2, 2013 – DARPA awards Moderna $25M for mRNA development – TIMELINE
- September 6, 2017 – 9 month after CEPI’s launch they call for “new vaccine platform technologies” – TIMELINE
- October 16, 2018 – BioNTech CEO predicts mRNA tech for rapid vaccine development – TIMELINE
- April 23, 2019 the Johns Hopkins Center for Health Security publishes a report titled “Vaccine Platforms: State of the Field and Looming Challenges” – TIMELINE
- September 19, 2019 – Trump signs EO to “Modernize Influenza Vaccines” – TIMELINE
- October 29, 2019 – Health experts discuss “roll out” of a Universal Flu Vaccine – TIMELINE
- April 12, 2020 – Bill Gates: a vaccine for 7 billion people is the only solution – TIMELINE
Pfizer approval
The FDA granted FULL APPROVAL for Pfizer-BioNTech’s Comirnaty on August 23, 2021. On only March 17, 2020 the two companies announced their collaboration to develop the mRNA vaccine. This is alarming given all the glaring issues found in the Pfizer documents used to grant this approval.
Moderna approval
On January 31, 2022 the FDA granted FULL APPROVAL for Moderna‘s COVID-19 vaccine which they called Spikevax – Spikevax is the name of the mRNA platform for “COVID-19” – no matter what variant code they insert! It was only on February 21, 2020, following Moderna received funding from CEPI, and that mRNA-1273 was listed as a product candidate in pre-clinical development just 23 months before full approval. Unprecedented!
I’ve documented aspects of the history of Big Vaccines on this website, on this page I’d like to focus on information that builds the picture around the new mRNA vaccine platform – or Next Generation Vaccines.
I expect they’ll change many of the childhood vaccines over to mRNA – unless we stop them!
mRNA vaccine platform data points in reverse chronological order
2025
August 11, 2025 – ICAN press release: ICAN Obtains Further Disturbing Details About the Government’s Self-Spreading Vaccine Program – READ
- Department of Defense (DOD) is continuing its drive to develop “synthetic immune systems.”
2024
October 1, 2024 – Dr Trozzi: Japan: The new testing ground of self-replicating RNA [REPLICON] – an ALERT Bridle, Makis and Trozzi -A radical genetic injection that offers no benefit, will produce death and disease, and may trigger a global catastrophe. – READ & WATCH, at the September 24-28, 2024 – 6th International Crisis Summit in Tokyo
- “Once a small amount of self-replicating RNA enters human cells, those cells will produce even more RNA, which in turn triggers the production of more spike proteins. This toxic snowball effect could result in widespread transfection, where the self-replicating RNA spreads from injected individuals to uninjected ones. We are venturing into unknown territory, and the potential consequences are deeply concerning.”
- Sept 3, 2024 -via Aussie17: Japanese Dr. Miki Gibo expresses concerns about Replicon mRNA vaccines that will be launching this fall – concern that it might spread even to the unvaccinated – TWEET
- Arab News: A group of Japanese doctors and university professors, united under the name “The United Citizens for Stopping mRNA Vaccines,” have raised serious concerns about the Replicon vaccine. This vaccine, scheduled to be administered to Japanese citizens in October, is being opposed due to what they branded as identified risks.” READ
February 14, 2024 – ICAN Uncovers a Potential Next-Level Threat: “Inhalable” Self-Spreading Vaccines that Spread Like a Virus – READ new vaccine deployment technology
- A new class of “encrypted RNA” vaccines are being developed, spread without a persons knowing or informed consent
- 2 companies have received millions $ federal government tax payer money! Currently animal studies for SARS-CoV-2 – Grant funded July 2021 in partnership with VxBiosciences Inc. – REF
- ICAN’s attorneys have already sent legal demands to all government agencies involved
February 2, 2024 – European Parliament Question: Lobbies calling for mRNA vaccines not to be classified as gene therapy – READ, ARCHIVE, Kangaroo Group (Moderna & BioNTech speak) – ARCHIVE, PDF, CREDIT
- On 17 January 2024, BioNTech and Moderna lobbyists (Kangaroo Group) hosted a lunch in the European Parliament with Members to discuss the forthcoming revision of the pharmaceutical legislation i.e. “European Commission’s proposal for a reform of the EU General Pharmaceutical Legislation (GPL)”.
- “Their main objective was to change the legislation’s definition of ‘gene therapy’. At present, mRNA vaccines (like the COVID-19 vaccine) are to be classified as gene therapy. However, they felt that only products that modified the genome should be classified as gene therapy.” – REF
- “As demonstrated during the Covid-19 pandemic, mRNA is an agile and flexible platform technology, enabling faster provision of vaccines and treatments to patients….it is important that the GPL provides a clear and broad scope for the definition of Platform Technologies, as well as a clear demarcation between Gene Therapies Medicinal Products that alter human genomes, and those that do not (e.g. mRNA)….to ensure global regulatory alignment.” – Kangaroo group – REF
2023
November 1, 2024 – PRESS RELEASE: Thirty-One mRNA Leaders Launch First Global Organization Dedicated to Advancing mRNA Medicines – READ, Promo video – WATCH, The mRNA drug industry strikes back – CREDIT, TIMELINE
- “31 biotechnology, biopharma and life science companies and educational institutions at the forefront of mRNA and next-generation encoding RNA therapeutics and vaccine development launched the Alliance for mRNA Medicines (AMM).”
- AMM is the first and only scientific and policy organization singularly focused on advancing and advocating for global mRNA innovation and the sector’s top policy priorities before legislative and regulatory bodies in North America, Europe and Asia. The Alliance issued its announcement at the 11th International mRNA Health Conference in Berlin, Germany.
- Neither Moderna nor Pfizer are on the members list (as of Aug 2025), but BioNTech is – HERE, initail list ARCHIVE
- Australia is replresented by CSL (founding member), Australian National University, Uni Queensland,

Advancing mRNA and next-generation RNA therapies
November 2203 – Trends in Biotechnology: Rise of the RNA machines – self-amplification in mRNA vaccine design – Comes et al – READ
- ABSTRACT: “The next step in mRNA vaccine design is the application of viral-based self-amplifying mRNAs (replicons) that provide long-lasting humoral and cellular immune responses upon single, low-dose immunization. Replicons encode their own replication machinery to boost their copy numbers directly after administration in target cells, which dramatically lowers the required initial mRNA dose and may consequently reduce adverse effects in individuals. Recent advances in mRNA formulation using lipid or solid nanoparticles create opportunities for novel applications for replicons such as mucosal delivery. Replicon vaccines hold potential as a platform technology when safety aspects are properly addressed.”
November 28, 2023 -Meiji Holdings Co. Ltd announces KostaiveTM, Self-Amplifying mRNA Vaccine against COVID-19, Approved for Manufacturing and Marketing from MHLW in Japan – for ARCT-154 developed by CSL and Arcurus Therapeutics – PDF
November 28, 2023 – CSL Press Release: Japan’s Ministry of Health, Labour and Welfare Approves CSL and Arcturus Therapeutics’ ARCT-154, the first Self-Amplifying mRNA vaccine approved for COVID in adults – READ, Nature – READ
- World’s first Self-Amplifying messenger RNA (sa-mRNA) COVID-19 Vaccine, This will become known as “replicon RNA vaccine”
October 2, 2023 – The Nobel Prize in Physiology or Medicine 2023 was awarded jointly to Katalin Karikó and Drew Weissman “for their discoveries concerning nucleoside base modifications that enabled the development of effective mRNA vaccines against COVID-19” – READ, TIMELINE
August 22, 2023 – HHS PRESS RELEASE: Project NextGen Awards Over $1.4 Billion to Develop the Future of COVID-19 Vaccines and Therapeutics – READ, CREDIT
- “$1.4 billion for Project NextGen to support the development of a new generation of tools and technologies to protect against COVID-19 for years to come”
- Project NextGen, is a $5 billion initiative led by ASPR’s BARDA in partnership with NIAID
May 15, 2023 – News: Clinical trial of mRNA universal influenza vaccine candidate begins – TWEET, READ, Gateway Pundit – READ, – Sept. 2019 – TIMELINE, October 2019 – TIMELINE
- “This Phase 1 trial will test the experimental vaccine, known as H1ssF-3928 mRNA-LNP, for safety and its ability to induce an immune response.” …”The trial will enroll up to 50 healthy volunteers aged 18 through 49″ [age group not at risk!] – Clinical Trial NCT05755620 – READ
- “The trial will enroll up to 50 healthy volunteers aged 18 through 49. Three groups of study participants (10 participants each) will be vaccinated with 10, 25 and 50 micrograms of the experimental vaccine, respectively. After evaluation of the data to determine an optimum dosage, an additional 10 participants will be enrolled to receive the optimum dosage. The study also will include a group of participants who will receive a current quadrivalent seasonal influenza vaccine.” [As always NO Placebo control]
- “The Centers for Disease Control and Prevention estimates that between 2010 and 2020, between 12,000 and 52,000 people died of flu in the United States annually” – [Note they did not say “influenza” but flu, which captures every flu-like illness including pneumonia, most are not “influenza”]
- In 2016 the NIAID created the Collaborative Influenza Vaccine Innovation Centers (CIVICs) – to support NIAID’s initiative to develop a universal influenza vaccine – WEB,
April 25, 2023 – Epoch Times: New Vaccine Printers to Produce COVID-19 mRNA Shots on Demand- READ, Chief Nerd – CREDIT
- “Published on April 24 in Nature Biotechnology, “Massachusetts Institute of Technology (MIT) scientists said their current vaccine printer prototype can produce 100 thumbnail-sized patches in 48 hours, with the potential to be scaled up to generate up to hundreds of vaccine doses a day.”
- April 24, 2023 – Nature Biotechnology: A microneedle vaccine printer for thermostable COVID-19 mRNA vaccines by Straeten et al – READ
April 18, 2023 – Chief Nerd: The Coalition Advocating for Adequately Labeled Medicines (CAALM) recently filed a petition requesting the FDA update product labeling for the Pfizer and Moderna COVID vaccines to better reflect their true safety and efficacy. – CREDIT, FDA reply Letter – PDF, ARCHIVE
- “FDA authorization and licensure standards for vaccines do not require demonstration of the prevention of infection or transmission…Similarly, a vaccine can meet the EUA standard without any evidence that the vaccine prevents infection or transmission.”
April 11, 2023 -Washington Post: White House launching $5 billion program to speed coronavirus vaccines – “Project Next Gen” – READ
- Dr Meryl Nass: “…this is the project that Drs. Fauci, Morens and Taubenberger called for in their January 2023 article, admitting that existng flu, RSV and COVID shots work poorly.” – READ
- Global Center for Health Security: “‘Project Next Gen’ would succeed ‘Operation Warp Speed’ with a mission to develop next-generation vaccines and therapies” – REF
April 1, 2023 – Malone Substack: RNA Vaccine Clinical Trials – READ
March 15, 2023 – Biocentury: Warp speed isn’t fast enough: the need for variant-proof therapeutics By Tevi Troy and Ariel Weinberger, Autonomous Therapeutics – READ, ICAN- CREDIT
- “This next-generation RNA, which we aim to advance at Autonomous Therapeutics Inc., is designed to encode an artificial immune system that can detect and eliminate every variant of a viral family. We have developed encrypted RNA candidates with potential variant-proof efficacy against a wide range of pathogens, from coronaviruses to influenza and RSV. These can be made inhalable and shielded from human immune systems to enable safe and long-term prophylaxis. (Emphasis added.)” –
February 24, 2023 – CDC ACIP meeting: COVID-19 vaccines: future directions – PDF, MEETING, ARCHIVE [This vaccine starts and assures the “vaccine platform” can be used for all future vaccines, so they have to keep pushing it into every arm]
- COVID-19 message fatigue challenges for vaccine uptake! – they aim to keep it simple in future!!! [Science?]
- “Current COVID-19 vaccine recommendations are complex” – “Simple recommendations are easier to communicate, which may improve uptake“
- Uptake of current bivalent vaccine is low
- SARS-CoV-2 continues to evolve, but recent virus evolution has not led to large population-level surges in cases or hospitalizations” [but they have to find something to justify continuing to push the vaccine]
- Most adults have a had prior infection [natural immunity], prior vaccination [transient antibody production], or both, [no mention of kids]
- “A plan for a fall booster dose could provide added protection, at a time when many would be ~1 year from last dose” [i.e. lets guess and assume it could benefit!]
- “Doctor’s offices and clinics were the most trusted place for parents to have their child receive a COVID-19 vaccine”
February 20, 2023 – Aaron Siri Substack | Injecting Freedom: mRNA Vaccines are “Vaccines” – Like it or not, mRNA vaccines are no less a vaccine than other vaccines – READ
- If preventing infection and transmission is necessary to call a product a vaccine, then pertussis vaccine cannot be called a vaccine, nor can diphtheria and tetanus and polio.
- Toxoid vaccines only generate antibodies to the “formaldehyde-treated toxins”, they don’t generate immunity to the bacterial pathogen! Thus these created super-spreaders
- As per CDC & WHO pre-covid definitions a “vaccine” was defined as a “product that produces immunity,” and the term “immunity” is determined by “the presence of antibodies in the blood.” Nothing about preventing infection or transmission – so mRNA vaccines fit their definitions.
- Just like mRNA vaccines attenuated Frankenstein rubella viruses, unlike anything found in nature, take over the cellular machinery of the cell to replicate this Frankenstein virus! [wow I didn’t know that!] Plus “each dose of rubella vaccine contains a vast amount of human DNA and cellular debris from the aborted fetal cell line“
- The common denominator is that they all artificially stimulate the immune system in the attempt to generate antibodies to a disease. Most are injected deep into muscle tissue, an unnatural route of exposure, and cause a sustained immune response”
- All products labeled “vaccine” go through the biologic division of the FDA, [or equivalent for other country regulators i.e TGA Advisory Committee on Vaccines – yet inTGA regulations they do not define what constitutes a vaccine]
- The Pfizer-BioNTech and Moderna mRNA vaccines trials “were incredibly more robust than the trials for childhood vaccines”. [The public doesn’t realise this!] “…most childhood vaccines had only days or weeks of safety review; typically far, far less than 30,000 participants; and virtually never had a placebo control.”
- Not only can you NOT sue any COVID-19 vaccine manufacture for harms, any vaccine on the US childhood schedule, you can’t sue the manufactures either – Thanks to the 1986 Act.
- Prediction the ” CDC and FDA will declare (as it has done with certain other vaccines) that Covid-19 was brought to heel because of the Covid-19 vaccines.”
- “…all vaccines that fall into the same economic and regulatory model that permits pharma to harm people with impunity with these products.”
February 11, 2023 – Biocentury: FDA crafting platform technology program – New platform tech designation could enable faster, more predictable product development – READ, CREDIT
- It may also now be possible to develop plug-and-play platform technologies that are resistance-proof and rapidly adaptable to any viral threat. And the regulatory process for applying these platforms to new viral indications may soon be streamlined — due to the new advanced platform technology designation provided by the PREVENT Pandemics Act, passed by Congress in the recent 2023 omnibus appropriations bill.
February 10, 2023 – Brownstone Institute: The CDC Lied: The mRNA Wasn’t Meant to “Stay in the Arm” – BioNTech’s platform specifically targets the lymph nodes – the Lipid Nanoparticles make this possible (along with all other parts of the body) – READ
- January 26, 2023 – Chief Medical Officer of BioNTech, Özlem Türeci (BioNTech’s joint owner with her husband) presentation – The future of rna-based technology – TWEET
- “Bringing mRNA to the right cells at the right places.” The deltoid is not the right place; the lymph nodes are. – mRNA is destined for the lymph nodes – and always has been
January 26, 2023 – FDA’s VRBPAC meeting three Big Pharma companies got to present data on their vaccine platforms – TIMELINE
January 20, 2023 – TGA: Australia’s Therapeutic Goods Administration (TGA) refered to Modern’s Spikevax as a “Genetic vaccine platform” – TIMELINE
January 11, 2023 – Cell: Rethinking next-generation vaccines for coronaviruses, influenzaviruses and other respiratory viruses – Morens, Taubenberger and Fauci – READ, TIMELINE
2022
December 22, 2022 – US Senate Committee on Health, Education, Labor & Pensions: Senate Passes Murray-Burr PREVENT Pandemics Act – READ, WATCH, ICAN – CREDIT, TIMELINE
- “Congress tucked a law, the PREVENT Pandemics Act, into the 2023 omnibus appropriations bill to facilitate new vaccine deployment technology under a section dedicated to Platform Technologies that supports the “development and review of new treatments and countermeasures that use cutting-edge, adaptable platform technologies that can be incorporated or used in more than one drug or biological product.”
- Health Extenders and Strengthening Public Health Act of 2022 – section 2503 Platform technologies- PDF, ARCHIVE
- Sen. Murray Calls for Congress to Pass Bipartisan PREVENT Pandemics Act In End-of-Year Package – WATCH

November 24, 2022 – Science: A multivalent nucleoside-modified mRNA vaccine against all known influenza virus subtypes – Arevalo, Scott Hensley et al – READ, NIAID – READ
September 29, 2022 – Malone Substack: FDA is using the COVID-19 Vaccines as a “Platform Technology” for mRNA Vaccine Trials – This shows shocking idiocy and malfeasance on the part of the FDA, as well as complete regulatory capture. – READ
- “CBER decided that going forward, with new mRNA vaccine trials, ONLY the product (the final formulation) should be tested as long as same manufacturing process and LNP are used. This is despite the fact that CBER did not do complete biodistribution or toxicity studies on these products, as discovered in the FOIA Japanese pre-clinical package and the US court ordered document release.”
- “CBER has determined that bio-distribution studies on new mRNA vaccines using this “platform technology” will not have to be redone , even though they were not properly evaluated in the first place.”!
- “What this all means is that using these flawed pre-clinical trials to support a platform technology was PLANNED from the beginning. By not focussing on the payload of the vaccines, but instead relying on the generic formulations prior to initiating clinical trials, this has allowed CBER (as well as Moderna, and Pfizer/BioNTech) to transfer these highly flawed pre-clinical data packages to all upcoming mRNA vaccine trials for new vaccine products!”
- [so much for FDA’s “rigorous scientific and regulatory processes”]
August 11, 2022 – BioRxiv preprint: A single-administration therapeutic interfering particle reduces SARS-CoV-2 viral shedding and pathogenesis in hamsters – Chaturvedi, Leor Weinberger et al – READ, published PNAS Sept 8, 2022 – READ – self-spreading vaccine – NIH HIV – GRANT
July 1, 2022 – Reuters: FDA will not require clinical trial data to authorize redesigned COVID boosters – READ
June 22, 2022 – Brownstone Institute: The FDA’s “Future Framework” for Covid Vaccines Is a Reckless Plan – Toby Rogers – READ
June 28, 2022 – FDA VRBPAC meeting – WATCH, Meryl Nass notes – READ, CHD – ARTICLE
- Peter Marks claim a [variant] switch will be made based on immunobridging. At an April 6, 2022 meeting “it was widely acknowledged that immunobridging (antibody titers) were not a reliable marker of immunity, and therefore actual clinical trials to measure efficacy would have to be done since the U.S. Food, Drug and Cosmetic Act requires an efficacy evaluation to approve a drug or vaccine.” – they ignored this
- In the end te question for a vote was whether to add an (unspecified) Omicron component to COVID boosters in the U.S.? – Two No’s, 19 yeses, no abstentions. Bernstein and Offit voted no. – bivalent vaccine approved without clinical trial data
- Note SAFE was mentioned “provaccine advocacy group” who formed in 2020! – their website first archived April 2021, constructed by political consulting Superior Blue! [who exactly is this anti-antivaxxer group?]
June 1, 2022 – CHD: Will FDA Allow Pfizer, Moderna to Skip Clinical Trials for Future COVID Vaccines? – FDA’s VRBPAC to vote on “Future Framework” – a scheme that would allow Pfizer and Moderna to “reformulate” COVID-19 mRNA vaccines in perpetuity, without conducting clinical trials on the new vaccines – READ
May 2022 – Gates Notes: How to prevent the next pandemic – by Bill Gates – Vaccinate the world in six months – READ
April 6, 2022 – FDA introduces “Future Framework” for re-coding mRNA vaccines, no need for trials! – TIMELINE
- The “Future Framework,” a FDA regulatory scheme that would allow Pfizer & Moderna to “reformulate” COVID-19 mRNA vaccines in perpetuity, without conducting clinical trials on the new vaccines – REF
March 31, 2022 – NIH: NIH begins clinical trial evaluating second COVID-19 booster shots in adults – Study includes multiple variant vaccines – READ, TIMELINE
“We are looking beyond the Omicron variant to determine the best strategy to protect against future variants,” “This trial will help us understand if we can use prototype and variant vaccines alone or together to shift immune responses to cover existing and emerging COVID-19 variants.”
Anthony S. Fauci – NIAID Director
February 15, 2022 – Viruses: mRNA Vaccine Development for Emerging Animal and Zoonotic Diseases – Le et al (China) – READ
- “Recently, the successful application of mRNA vaccines against COVID-19 has further validated the platform and opened the floodgates to mRNA vaccine’s potential in infectious disease prevention, especially in the veterinary field.”
January 31, 2022 – Emerging Microbes & Infections: WHO informal consultation on regulatory considerations for evaluation of the quality, safety and efficacy of RNA-based prophylactic vaccines for infectious diseases, 20–22 April 2021 – Liu et al – READ, CREDIT —Updates on global development of prophylactic mRNA vaccines for infectious diseases
2021
December 7, 2021 – WHO: Evaluation of the quality, safety and efficacy of messenger RNA vaccines for the prevention of infectious diseases: regulatory considerations – Annex 3 – READ, ARCHIVE, DOCUMENT
- Justification for mRNA plaftorm as front runner vaccine technology is SPEED – mass production for whatever “emerging” pathogen they find!
November 29, 2021 – CEPI: Developing pandemic-busting vaccines in 100 days – The world can face down the next Disease X with a new vaccine in just 100 days. – READ, CREDIT
- Put this into context with proposed new WHO Pandemic powers, WEF push of Disease X in 2024, vaccine mandates, digital ID’s etc
…we were preparing for covid-like diseases. You may even call COVID as the first Disease X, and it may happen again…
WHO Director General Tedros @ WEF Davos 2024
November 9, 2021 – Cell Journal: Identification of a therapeutic interfering particle—A single-dose SARS-CoV-2 antiviral intervention with a high barrier to resistance – Chaturvedi, Weinberger et al – READ, READ, ICAN – CREDIT
- Therapeutic interfering particles (TIPs) inhibit SARS-CoV-2 in cell culture…Intranasal administration in hampsters
- Gladstone Institutes & VxBiosciences – Leor Weinberger: A New Class of Antiviral Therapy Could Treat COVID-19 – READ
November 7 2021 – Policy Exchange UK: Bill Gates speaks to Rt Hon Jeremy Hunt MP in exclusive interview – WATCH, TIMELINE
- Gate’s recounts what wasn’t achieved in this COVID-19 pandemic
“Making vaccines cheap [mRNA platform], building big factories, eradicating the flu, getting rid of the common cold, making vaccines jut a little patch you put on your arm, things that will be incredibly beneficial even in years when we don’t have pandemics“
Bill Gates, setting the stage for future “preparedness”
October 27, 2021 – Nature: An mRNA vaccine industry in the making – The technology could form the basis of a new generation of vaccines for diseases such as HIV/AIDS and malaria. – READ
- “The course of the COVID-19 pandemic was changed in late 2020 with a vaccine based on messenger RNA (mRNA) [What about J&J and AstraZenaca vaccines?], an unprecedented technology that has proved remarkably successful in protecting against the virus [Define “proven” and define “protecting!!!]] Not only is mRNA set to help get the world past the current crisis, it’s also generating hope for a whole new generation of vaccines that could protect people from everything from HIV/AIDS to malaria.”
October 18-22, 2021 – WHO: 74th Expert Committee on Biological Standardization (ECBX) – meeting outcomes – READ, ARCHIVE, PDF
- Adopted at this meeting: Evaluation of the quality, safety and efficacy of messenger RNA vaccines for the prevention of infectious diseases: regulatory considerations: – DOCUMENT
October 19, 2021 – Mainichi: Japan firm starts clinical trial for next-generation COVID-19 vaccine requiring smaller dosage – READ, Stanley Plotkins is on the Advisory board – READ
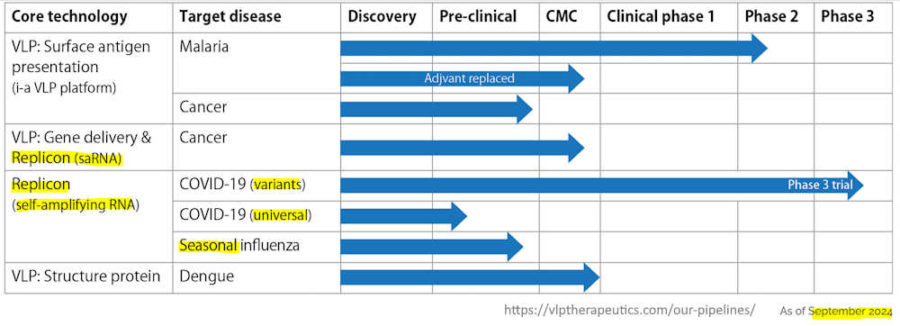
- VLP Therapeutics Japan uses new “replicon” technology, a next-generation messenger ribonucleic acid (mRNA) that self-replicates inside the recipient’s body after vaccine administration. It reportedly requires a dosage of only 1/10 to 1/100 of the mRNA vaccines that have been put to practical use, and can be supplied in large quantities over a short time.
When new infectious diseases spread in future, this will serve as domestically-produced fundamental technology that allows us to react swiftly.
Wataru Akahata, head of VLP Therapeutics Japan
- September 29, 2022 -Phase 2 clinical trial of self-amplifying “replicon” mRNA COVID-19 booster dose – READ
- December 20, 2023 -Phase 3 clinical trial of self-amplifying “replicon” RNA COVID-19 booster targeting Omicron XBB.1.5 – READ
- VLP Therapeutics is a Maryland, US-based biotech company co-founded in 2013 by Drs. Wataru Akahata et al. VLP Therapeutics Japan, Inc. (VLPT Japan), founded in 2020 by Dr. Wataru Akahata originally as a wholly owned subsidiary of US-based VLP Therapeutics, Inc., is a Tokyo-based biotech company.
- November 29, 2018 – Wataru Akahata, Ph.D. Recognized by the Japanese Government as 2018 Significant Researcher – REF
- Part funding from Bill & Melinda Gates Foundation, the Wellcome Trust via GHIT Fund – READ
October 2021 – Moderna already have a “COVID + Flu vaccine” in preclinical development – there are many more vaccines in various stages of development – ARCHIVE, READ
September 14, 2021 – Nature: The tangled history of mRNA vaccines – Hundreds of scientists had worked on mRNA vaccines for decades before the coronavirus pandemic [conveniently] brought a breakthrough – “In late 1987, Robert Malone perfored a landmake experiment…” – READ, ARCHIVE
- “Those experiments were a stepping stone towards two of the most important and profitable vaccines in history: the mRNA-based COVID-19 vaccines given to hundreds of millions of people around the world. Global sales of these are expected to top US$50 billion in 2021 alone.”
July 28, 2021 – Pfizer Second Quarter 2021 Earnings Teleconference – PDF
- Pfizer already had a flu vaccine ready for testing which they claim is based on “proven” mRNA vaccine platform.
April 20, 2021 – WHO informal consultation on regulatory considerations for evaluation of the quality, safety and efficacy of RNA-based prophylactic vaccines for infectious diseases – PDF, TIMELINE,
- [And so the mRNA vaccine platform is given the green light…thanks to the rush “need” of COVID-19]
- ““Evaluation of the quality, safety and efficacy of mRNA prophylactic vaccines for infectious diseases: regulatory considerations” (hereinafter “WHO RNA document”) was published in order to invite public comments from 22 December 2020 to 31 January 2021″
- “The COVID-19 pandemic and the corresponding mRNA vaccine development against COVID-19 made it clear that the document needed to be developed as rapidly as possible in order to provide special considerations for this class of vaccines.”
March 11, 2021 – Uni California San Fransisco (UCSF): Viruses Mutate, But Treatments Are Static. Is There a Way to Change That? – READ, – inhalable self-spreading vaccines – CREDIT, TED talk – WATCH
- Therapeutic Interfering Particles (TIPs) “are engineered mutants of the virus themselves, little snippets of similar DNA. They outcompete the virus for replicating material, so instead of spreading the virus, the virus spreads the harmless mutant. As the TIPs spread, the virus fails to replicate further, decreasing the viral load.
- “After testing the TIP in animal models, Weinberger is preparing his first clinical trial for later this year, which will be done with HIV positive patients who also have a terminal illness
- “Overall, Weinberger said, the U.S. scientific community is less comfortable talking about anything that feels like genetic engineering, especially changes that can be passed onto other people. There is more enthusiasm for this type of solution in Africa, where nearly 20 million people have HIV, and where in 2019, about 150,000 children contracted the virus.” [Same playbook, first target third world Africa with suspect HIV then bring it to the rest of the world]
- They pivoted to COVID-19 July 2021
“We can make vaccines fairly quickly. That’s not the problem,” …. “The problem is distributing them and remaking them – where with TIPs, they take care of that on their own.”
Leor Weinberger
March 5, 2021 – Australian TGA’s Access Consortium: Points to consider for strain changes in authorised COVID-19 vaccines in an ongoing SARS-COV-2 Pandemic – READ, TIMELINE
- “Regulatory Authorities do not consider an updated coronavirus vaccine to be an entirely novel product with the resulting requirement for lengthy full-blown clinical studies.” They consider “a regulatory approach like for seasonal updates for influenza vaccines can be taken”
- “Since an updated vaccine variant will build on a previously authorised parent version with established quality, safety and efficacy; from a public health perspective, it may be justifiable to roll out the new vaccine candidate already in parallel with the previous version in absence of clinical immunogenicity and safety data while these studies are ongoing.””
January 7, 2021- Vaccines: Development of mRNA vaccines: scientific and regulatory issues – Knezevic et al – READ, CREDIT
- “there remain gaps in our understanding of the mechanism of action of mRNA vaccines, as well as their long-term performance in areas such as safety and efficacy.”
- “New initiatives are ongoing at WHO to arrive at a broad consensus to formulate international guidance on the manufacture and quality control, as well as nonclinical and clinical evaluation of mRNA vaccines..”
- “This paper reviews the technologies and processes used for developing mRNA prophylactic vaccines, the current status of vaccine development, and discusses the immune responses induced by mRNA vaccines.”
2020
December 18, 2020 – Nature: The lightning-fast quest for COVID vaccines — and what it means for other diseases – The speedy approach used to tackle SARS-CoV-2 could change the future of vaccine science – READ, ARCHIVE
- “The basic research on DNA vaccines began at least 25 years ago, and RNA vaccines have benefited from 10–15 years of strong research,”
December 9-10, 2020 – WHO: 73rd meeting WHO Expert Committee on Biological Standardization (ECBS) – meeting outcomes – READ, PDF, ARCHIVE
October 27, 2020 – TED MED (unlisted): Can we create vaccines that mutate and spread? Virologist Leor Weinberger – WATCH, READ, SOURCE, CREDIT
August 28, 2020 – Canadian Cattlemen Magazine: Vet Advice: mRNA transforms science behind vaccine development – READ
- “SARS-CoV-2, the agent implicated in the ongoing COVID-19 pandemic, pried doors open for a new generation of vaccine development, the newest being messenger RNA (mRNA) technology.”
- “Globalization [population growth] and climate change increase the likelihood of new patterns of emergence and spread of human and livestock viruses.” [No mention on increasing numbers of BSL4 labs!]
- Professor Bekeredjian-Ding of Germany’s Paul Ehrlich Institute makes the following comments about the mRNA technology platform:
- “mRNA vaccines against infectious diseases are new. Much of the research to date on mRNA vaccines is related to cancer prophylaxis and treatment. An mRNA vaccine has never been registered for infectious disease.
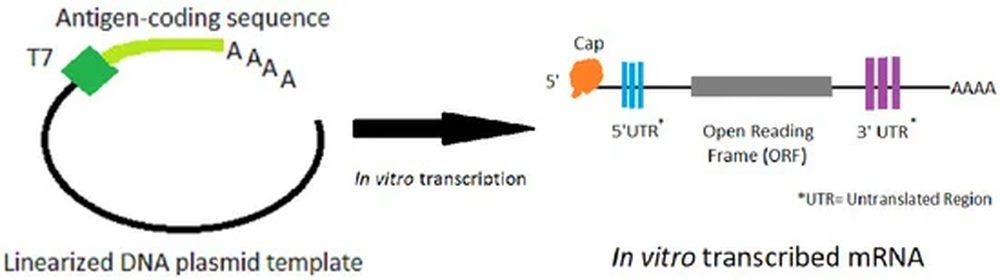
- “A major advantage of mRNA vaccines is that RNA can be produced in the laboratory from a DNA template using readily available materials, less expensively and faster than conventional vaccine production, which can require the use of chicken eggs or other mammalian cells.”
- Although mRNA vaccine technology has not been extensively tested, preclinical and early clinical trials show promise using animal models. Research on mRNA technology is about to expand exponentially. The ship has already sailed.
- “mRNA vaccines against infectious diseases are new. Much of the research to date on mRNA vaccines is related to cancer prophylaxis and treatment. An mRNA vaccine has never been registered for infectious disease.
August 24-28, 2020 – WHO: 71st meeting of the WHO Expert Committee on Biological Standardization (ECBS) – Executive Summary: Main outcomes of the meeting – READ, ARCHIVE, PFD
- “Although no RNA-based vaccines have yet been approved for human use, this platform technology has the potential to facilitate the rapid development of vaccines against priority pathogens in public health emergencies” – curiously they refered to the vaccines as “SARS-CoV-2 mRNA vaccines” not COVID-19 vaccines.
- ECBS supported the development of a document on the “regulatory considerations for the evaluation of mRNA vaccines
- They noted that currently there are no guidelines specifically for SARS-CoV vaccines or more generally for vaccines based on RNA platforms.
- “In addition, despite no evidence to date that SARS-CoV-2 candidate vaccines cause vaccine-associated enhanced respiratory disease, an analogous effect has been reported in a SARS-CoV-1 mouse model and rigorous assessment of this would be required.”
June 12, 2020 – Gladstone Institutues: Becoming Equipped for Future Pandemics – READ, CREDIT
- “The sudden emergence—and extremely rapid spread—of COVID-19 is an important reminder of the devastating impact that a single pathogen can have on the human body, and on society….
[U]sing viruses as therapeutics,…The first strategy, led by Senior Investigator Leor Weinberger, PhD, is to use the virus’s tricks against it. To do so, he is engineering therapeutic interfering particles, or TIPs. These particles hijack the virus’s circuitry and convert infected cells from virus-producing factories into therapy-producing factories.”
April 12, 2020 – BBC Breakfast: Coronavirus: Bill Gates interview @bbcbreakfast4365 – [investor in vaccines etc and not a health expert] – WATCH, TIMELINE
“…the thing that will get us back to the world that we had before coronavirus is the vaccine and getting that out to all 7 billion people”
“…so we’re going to have to take something that usually takes 5 to 6 years [to develop] and get it done in 18 months. There is an approach called an RNA vaccine…that looks quite promising…unfortunately the schedule for the [conventional vaccine approach] will probably not be as quick as the RNA platform, that we’ve been funding directly and through CEPI over the last decade.”
“…this is such an unprecedented, very tough thing to deal with. The people like myself and Tony Fauci are saying 18 months [to develop a vaccine], if everything went perfectly we can do slightly better than that, but there will be a trade off, we’ll have less safety testing than we typically would have, and so governments would have to decide do they indemnify the companies… we just don’t have the time to do what we would normally do“
Bill Gates
March 2, 2020, Moderna’s CEO Stéphane Bance, told President Trump that “we’re able to move very, very fast from a few phone calls to getting a vaccine made, ready for the clinic” – WATCH, TIMELINE
“…in only 42 days from the sequence of the virus, [we sent] our vaccine to Dr. Fauci’s team at the NIH“.Stéphane Bance – BioNTec CEO
Stéphane Bance – BioNTec CEO
March 2020 – TED TALK: Can we create vaccines that mutate and spread? by Leor Wienberger virologist – WATCH, CREDIT, Weinberger lab @ UCSF – WEB
- Weinberger served on the Bill & Melinda Gates Foundation Innovation review panel, and his research has been widely published in Science, Nature and Cell. -more bio – ARCHIVE, UCSF – HERE
- And cofounder of Autonomous Therapeutics, Inc. – ARCHIVE
- “Autonomous Therapeutics, Inc. is a preclinical biotechnology company founded in New York City in 2017 by inventors from Harvard Medical School and the University of California, San Francisco (UCSF)….We are developing the first therapies that evolve along with infectious diseases and cancers—to provide lifelong, ‘resistance-proof’ interventions….Imagine taking a vaccine drug once and never worrying about the flu again.” – REF
2019
October 29, 2019 – Milken Institute| Future of Health Summit: featuring Dr Anthony Fauci, and Dr Rick Bright discussed the scientific and technological prospects of an effective universal influenza vaccine – WATCH, TIMELINE
- The panel spoke about how to speed up the transition (@22min) from egg grown vaccines to introducing a new mRNA vaccine technology “that we haven’t given to anyone yet [5], a process that if “all works perfectly” could take as long as 10 years.
- By using an “entity of excitement” such as “an outbreak of novel avian virus” from “China somewhere” which would lead to “some kind of global event where many people were dying” and allow a “new mRNA vaccine to be” rolled out and “tested on the public.” [3]
“But it is not too crazy to think that an outbreak of a novel avian virus [or coronavirus!] could occur in China somewhere. We could get the RNA sequence from that, beam it to a number of regional centres, if not local, if not even in your home at some point and print those vaccines on a patch and self administer.“
Rick Bright
September 19, 2019 – President Trump signed an Executive Order (EO): “Modernizing Influenza Vaccines in the United States to Promote National Security and Public Health” – READ, TIMELINE
“[t]hese platform technologies include DNA, messenger RNA (mRNA), virus-like particles, vector-based, and self-assembling nanoparticle vaccines.”
May 28, 2019 – Task Force for Global Health: CEPI partners with Brighton Collaboration to support safety assessment of vaccine candidates against emerging infectious diseases – READ, TIMELINE
- The Brighton Collaboration & CEPI launch of the Safety Platform for Emergency vACcines (SPEAC) Project
- Initial focus includes MERS vaccine candidates.
April 23, 2019 – Johns Hopkins enter for Health Security: “Vaccine Platforms: State of the Field and Looming Challenges” – Report PDF, Vaccine Platform technologies – TIMELINE
February 19, 2019 – DARPA: A New Layer of Medical Preparedness to Combat Emerging Infectious Disease – ARCHVIE
- “DARPA has selected five teams of researchers to support PREventing EMerging Pathogenic Threats (PREEMPT), a 3.5-year program first announced in January 2018 to reinforce traditional medical preparedness by containing viral infectious diseases in animal reservoirs and insect vectors before they can threaten humans.”
- DARPA selects Autonomous Therapeutics – CREDIT
- Autonomous Therapeutics, Inc., under principal investigator Dr. Ariel Weinberger, leads a team made up of CSIRO Australian Animal Health Laboratory; Navy Medical Research Unit-2, funded directly by DARPA; University of California, Los Angeles; University of Chicago Medical School; and University of Texas Medical Branch. The team will study air-borne highly pathogenic avian influenza virus in birds and small mammals, and tick-borne Crimean-Congo hemorrhagic fever virus.”
2018
October 16, 2018 – Grand Challenges Meeting 2018: Spotlight Talk: BioNTech‘s founder and CEO Dr Ugur Sahin speaks – WATCH, TIMELINE
- Sahin said his company, BioNTech, “might be able to use its so-called messenger RNA technology to rapidly develop a vaccine in the event of a global pandemic”
April 10, 2018 – JAMA Network | Viewpoint: Novel Vaccine Technologies Essential Components of an Adequate Response to Emerging Viral Diseases – Graham, Fauci et al – READ, TIMELINE
February 28, 2018 – J Infectious Diseases: A Universal Influenza Vaccine: The Strategic Plan for the National Institute of Allergy and Infectious Diseases – Erbelding, Barney S Graham and Anthony S Fauci et a – READ
January 12, 2018 – Nature: mRNA vaccines — a new era in vaccinology – Pardi, Weissman et al – READ
- “Nucleoside-modified mRNA vaccines represent a new and highly efficacious category of mRNA vaccines. Owing to the novelty of this immunization platform, our knowledge of efficacy is limited to the results of four recent publications that demonstrated the potency of such vaccines in small and large animals.”
2017
September 6, 2017 – 9 month after CEPI’s launch they call for “new vaccine platform technologies” – TIMELINE, in order to meet the needs of Disease X!
September 23, 2017 – The Lancet: Safety and immunogenicity of a mRNA rabies vaccine in healthy adults: an open-label, non-randomised, prospective, first-in-human phase 1 clinical trial – Alberer et al (CureVac)- READ number NCT02241135
- “The primary endpoint was safety and tolerability. The secondary endpoint was to determine the lowest dose of CV7201 to elicit rabies virus neutralising titres [VNT] equal to or greater than the WHO-specified protective antibody titre of 0·5 IU/mL.” [So the WHO sets the antibody titre that is the surrogate for “immunity”, and preseumably why the gene technology product can be classified as a “vaccine”, it appears this figure comes from a 2004 WHO document – ARCHIVE]
- “This first-ever demonstration in human beings shows that a prophylactic mRNA-based candidate vaccine can induce boostable functional antibodies against a viral antigen when administered with a needle-free device, although not when injected by a needle-syringe. The vaccine was generally safe with a reasonable tolerability profile.”
July 26, 2017 – BioSpace News: CureVac Announces Publication In The Lancet Of First-Ever Human Proof-Of-Concept Study Investigating The Safety And Immunogenicity Of A Prophylactic mRNA Vaccine – READ
- CureVac AG, a fully-integrated biotechnology company pioneering mRNA-based drugs, today announced that the results of CureVac’s phase I clinical trial of its RNActive® prophylactic rabies vaccine was published in the peer-reviewed journal The Lancet. The study was the first in-human proof-of-concept clinical trial of a prophylactic mRNA-based vaccine. Subsequently, the mRNA drug substance encoding a rabies virus glycoprotein was given the International Nonproprietary Name (INN) Nadorameran by the World Health Organization (WHO) as first drug substance of this new class.
- Clinical Trial gov NCT02241135 RNActive® Rabies Vaccine (CV7201) in Healthy Adults – HERE
- July 25, 2017 – Lancet: Safety and immunogenicity of a mRNA rabies vaccine in healthy adults: an open-label, non-randomised, prospective, first-in-human phase 1 clinical trial – Alberer et al – READ
- “Between Oct 21, 2013 [ARCHIVE], and Jan 11, 2016, we enrolled and vaccinated 101 participants with 306 doses of mRNA” – so NO placebo, but 2 year followup [compared to 2-6 months for COVID-19 mRNA vaccines which ICAN forced a placebo control – TIMELINE]
June 2017 – Workshop convened by NIAID convened in June 2017 that gathered scientists from academia, industry, and government who developed criteria for defining a universal influenza vaccine, identified knowledge gaps, and delineated research strategies for addressing those gaps. The findings of that workshop were published in the journal Immunity and outlined four key criteria for a universal vaccine. – TIMELINE
- October 17, 2017 – Immunity Journal: The Pathway to a Universal Influenza Vaccine – Fauci et al – READ
- Barney Graham and Diane Post “felt that it was premature to promote any single vaccine platform or platforms at this point in time”
- “…it is critical in designing a universal influenza vaccine to understand better the basic B and T cell immune response to influenza infection and the effects of serial exposure to natural infection and vaccination. It is well recognized that the initial exposure to an influenza virus affects the antibody response to subsequent exposures to new strains. This concept, termed “original antigenic sin,” was proposed in 1960…”
- “Recent data provide strong epidemiologic evidence that infection with the influenza strain circulating during one’s childhood elicits a lifelong immunologic imprint that impacts responses to novel strains and can help protect against unfamiliar HA subtypes from the same phylogenetic group as the original infecting virus (Gostic et al., 2016). This phenomenon, termed “immunologic imprinting,” has important implications for public health because it affects responses to subsequent influenza infections and potentially to influenza vaccinations. Additionally, imprinting is likely to impact how individuals respond to different universal vaccine antigens. [!!!]
April 27, 2017 – Molecular Therapy: Preclinical and Clinical Demonstration of Immunogenicity by mRNA Vaccines against H10N8 and H7N9 Influenza Viruses – Bahl et al – READ, TIMELINE
- The paper, funded by Moderna Therapeutics, looked at the “Immunogenicity by mRNA Vaccines”, they concluded that “LNP-formulated, modified mRNA vaccines can induce protective immunogenicity with acceptable tolerability profiles” in mouse studies and a First-in-Human Phase 1 Study with 31 human subjects
February 17, 2017 – Munich Security Conference – Bill Gates transcript – READ, ARCHIVE, TIMELINE
Vaccines can be especially important in containing epidemics. But today, it typically takes up to 10 years to develop and license a new vaccine. To significantly curb deaths from a fast-moving airborne pathogen, we would have to get that down considerably—to 90 days or less.
The hope is that CEPI will enable the world to produce safe, effective vaccines as quickly as new threats emerge.
The really big breakthrough potential is in emerging technology platforms that leverage recent advances in genomics to dramatically reduce the time needed to develop vaccines… synthetic genetic material that instructs your cells to make a vaccine inside your own body. And the great thing is that once you’ve built a vaccine platform for one pathogen, you can use it again for other pathogens. You only need to substitute a few genes.
A highly lethal global pandemic will occur in our lifetimes.
Excerpts from Bill Gates speech
February 6, 2017 – DARPA News: DARPA’s Pandemic Prevention Platform (P3): Removing the Viral Threat: Two Months to Stop Pandemic X from Taking Hold – READ, ARCHIVE, CREDIT
- DARPA aims to develop an integrated end-to-end platform that uses nucleic acid sequences to halt the spread of viral infections in 60 days or less
- Over the past several years, DARPA-funded researchers have pioneered RNA vaccine technology
- January 12, 2017 – DARPA Tweet: Moderna has begun Phase I trials of DARPA-funded mRNA-1325 #Zika vaccine & cleared to begin trials of mRNA-1388 #Chikungunya #vaccine – TWEET
2017 – Guidelines on clinical evaluation of vaccines: regulatory expectations. In: WHO Expert Committee on Biological Standardization: sixty-seventh report. Geneva: World Health Organization; 2017: Annex 9 (WHO Technical Report Series, No. 1004 – PDF, referenced HERE
2017 – Automomous Therapeutics is co-founded by Dr Leor Weinberger and Dr. Ariel Weinberger – READ to develop “Lifelong, resistance-proof therapeutics” – READ, such as (inhalable) LNP delivery platforms – READ, following 2014 joint PAPER, CREDIT – Website – HERE
- “Imagine taking a vaccine drug once and never worrying about the flu again” –REF
- PRESS RELEASES since inception – READ
- Dr Leor Weinberger “is the inventor of the TIP evolving therapy concept, and the co-inventor of the Deletion Platform that engineers TIP therapeutics in just weeks.” – REF He is also Ariel’s brother!
- Leor Weinberger went on to start a separate company VxBiosciences around Dec 9, 2021, with their New “Hijacker” Therapy, with Gladstone Institutes – READ
- Patent for “generating a deletion library…” filed December 14, 2017 by Leor and Tim and Gladstone Institutes – READ, PDF
- By February 2019 their team includes CSIRO Australian Animal Health Laboratory scientists as reported in “DARPA selects Autonomous Therapeutics to support PREventing EMerging Pathogenic Threats (PREEMPT) initiative” PREEMPT is a 3.5-year program first announced in January 2018 – READ
- Dr. Timothy Notton is Chief Scientific Officer at Autonomous and co-inventor of Autonomous’ Encrypted RNA™ platform – REF
2016
November 9, 2016 – Biospace News: PhaseRx Announces Positive Safety Results From Large Animal Study With Hybrid mRNA Delivery Technology – READ
- This is an example of a LNP mRNA gene therapy product, which had great expectations treating life threatening disease in children, but the company website disapeared by 2018 – PhaseRX.com ARCHIVES
- The same type of platform technology concept of those they refer to in 2020 as “vaccines”
July 1, 2016 – UC San Diego, Human Vaccines Project Harness Advances in Machine Learning to accelerate the development of new vaccines and therapies – READ
- “…scientists have been experimenting with machine-learning tools for vaccine development for more than a decade. Machine learning typically involves ‘training’ a computer or robot on millions of actions so that the computer learns how to derive meaning from the data as time goes on”
March 15, 2016 – Merck Animal Health Press Release: Harrisvaccines Receives Production Platform Vaccine Licensure First of its Kind Granted by USDA – ARCHIVE, READ, READ, READ, Also see Edible Vaccines – HERE
- Merck Animal Health today announced that its recent acquisition, Harrisvaccines, has been granted licensure of its Prescription Product, RNA Particle (RP) vaccine platform from the U.S. Department of Agriculture (USDA). This first-of-its-kind USDA license approves the company’s innovative production platform and allows for the manufacturing of herd-specific, custom vaccines prescribed by a licensed veterinarian.
2014
July 15, 2014 – FDA Blog: Developing new tools to support regulatory use of “Next Gen Sequencing” data – READ
- …Such knowledge will contribute to advances in personalized medicine.
July 10, 2014 – Trends in Biotechnology – The case for transmissible antivirals to control population-wide infectious disease – Dr Timothy Notton, Dr. Ariel Weinberger & Leor Weinberger et al – READ, ICAN – CREDIT
- “These proposed antivirals, termed ‘therapeutic interfering particles’ (TIPs), are engineered molecular parasites of viruses that are designed to steal replication resources from the wild type virus. Depriving viruses of crucial replication machinery, TIPs would reduce viral loads.
- As obligate parasites, TIPs would transmit via the same risk factors and transmission routes as wild type viruses, automatically reaching high-risk populations, and thereby substantially limiting viral transmission even in resource-poor settings”
- TIPs are a new approach to overcoming universal barriers to infectious disease control, they… would function as transmissible antivirals. i.e. SELF-SPREADING
- In 2017 Ariel and Leor Weinberger co-founded Autonomous Therapeutics with Timothy Notton – their aim to develop “this technology “Lifelong, resistance-proof therapeutics” such as (inhalable) LNP delivery platforms – REF, READ
2013
October 2, 2013 – US Defence Advanced Research Projects Agency (DARPA) through its “synthetic biology” ADEPT: PROTECT Pandemic Prevention Platform program plans to have the “development and wide scale deployment of protective countermeasures” within 60 days from detection of an outbreak. – TIMELINE
- Moderna Therapeutics was awarded “up to $25 million to research and develop its messenger RNA therapeutics™ platform as a rapid and reliable way to make antibody-producing drugs to protect against a wide range of known and unknown emerging infectious diseases and engineered biological threats.”
May 31, 2013 – Science Magazine: Accelerating Next-Generation Vaccine Development for Global Disease Prevention – by Stanley Plotkins et al – READ
- This paper is the driver behind the Human Vaccine Project, a global non-profit, public-private partnership (PPP) – to accelerate new technology vaccines – TIMELINE
2012
September 20, 2012 – Press Release: Harrisvaccines Announces United States Department of Agriculture (USDA) licensure of the company’s swine flu vaccine, approved by the Department’s Center for Veterinary Biologics (CVB) for disease caused by swine influenza virus (SIV) H3N2 – The vaccine is the first to be licensed by the USDA CVB that utilizes RNA Particle Technology – READ, – TIMELINE
- This first licence for an RNA technology vaccine product in livestock sets the precedent for the approval of the RNA Particle (RP) technology Vaccine platform which happened in 2016. More info at Edible Vaccines – HERE
2011
September 16, 2011 – TED Talk | Richard Resnick: Welcome to the genomic revolution – WATCH, TIMELINE
- Genome sequencing is now so cheap and fast it will open up opportunities for personalised genome sequencing for health care, insurance and politics!
September 2, 2011 – Nucleic Acid Research: Generating the optimal mRNA for therapy: HPLC purification eliminates immune activation and improves translation of nucleoside-modified, protein-encoding mRNA – Kariko and Weissman et al – READ
2008
November 2008 – Molecular Therapy Journal: Incorporation of Pseudouridine Into mRNA Yields Superior Nonimmunogenic Vector With Increased Translational Capacity and Biological Stability – Kariko and Weissman et al – READ
- “…in its present form, mRNA is unfeasible for clinical use because of its labile and immunogenic nature.”
- “Even at higher doses, only the unmodified mRNA was immunogenic, inducing high serum levels of interferon-α (IFN-α). These findings indicate that nucleoside modification is an effective approach to enhance stability and translational capacity of mRNA while diminishing its immunogenicity in vivo.
- “Improved properties conferred by pseudouridine make such mRNA a promising tool for both gene replacement and vaccination.”
- To date the “use of mRNA has been mostly limited to vaccination in which antigen-encoding transcripts were administered in vivo or delivered to dendritic cells (DCs) ex vivo in order to induce cellular and humoral immune responses..”
- “Recent studies (2001-2004) have demonstrated that RNA activates cells of the innate immune system by stimulating Toll-like receptors (TLRs), specifically TLR3, TLR7, and TLR8…”
2008 – Handbook of Experimental Pharmacology: Vaccination with messenger RNA (mRNA) by Steve Pascolo (Uni Tuebingen, Germany) – READ, READ
- This is the second mRNA vaccine paper (pub 2005) authored by Pascolo, in 2004 he worked for CureVac- Messenger RNA-based vaccines – READ
- Pascolo works for same German univestity that hosted the first International mRNA Health Conference in 2014, mRNA herald a “New Era in Modern Medicine” – TIMELINE
2007
September 2007 – Current Opinions Drug Discover & Development: Naturally occurring nucleoside modifications suppress the immunostimulatory activity of RNA: implication for therapeutic RNA development by Katalin Karikó & Drew Weissman – READ, PDF
- “DNA and RNA stimulate the mammalian innate immune system by triggering a variety of sensors, including Toll-like receptors (TLRs). TLR9 signals upon exposure to DNA, while TLR3, TLR7 and TLR8 respond to RNA.”
- “Methylation of CpG motifs in DNA blocks TLR9 signaling…RNAs containing modified nucleosides, and thus lacking immune-activating properties, have potential importance in clinical applications.”
- “While it has been known for decades that ‘foreign’ nucleic acids, such as bacterial DNA and viral dsRNA, are potent adjuvants of the mammalian immune system… the mechanisms for nucleic acid recognition have only recently been discovered.”
2006
August 21, 2006 – PATENT: “RNA containing modified nucleosides and methods of use thereof“, filed by the University of Pennsylvania (UPenn) on behalf of the two “inventors” Katalin Kariko and Drew Weissman – TIMELINE, Patent – comprising pseudouridine or a modified nucleoside – READ
2005
August 2005 – Journal of Immunology: Suppression of RNA recognition by Toll-like receptors: the impact of nucleoside modification and the evolutionary origin of RNA – Kariko and Weissman – READ, CREDIT, Toll-like Receptor (TLR)
- Selected nucleosides in naturally occurring RNA are methylated
- “We show that RNA signals [innate immune system] through human TLR3, TLR7, and TLR8, but incorporation of modified nucleosides m5C, m6A, m5U, s2U, or pseudouridine ablates activity….
- The innate immune system may be able to detect RNA lacking nucleoside modification as a means of selectively responding to bacteria or necrotic tissue.
February 23, 2005 – Expert Opinion on Biological Therapy: Messenger RNA-based vaccines – Steve Pascolo (CureVac) – READ
- “RNA is the only molecule known to recapitulate all biochemical functions of life: definition, control and transmission of genetic information, creation of defined three-dimensional structures, enzymatic activities and storage of energy. Because of its versatility and thanks to several recent scientific breakthroughs, RNA became the focus of intense research in molecular medicine at the beginning of the millennium.”
- Presents “advantages and limits of the five different mRNA-based vaccination methods… that are paving the way for therapeutic and prophylactic drugs with mRNA as the active component.
2000
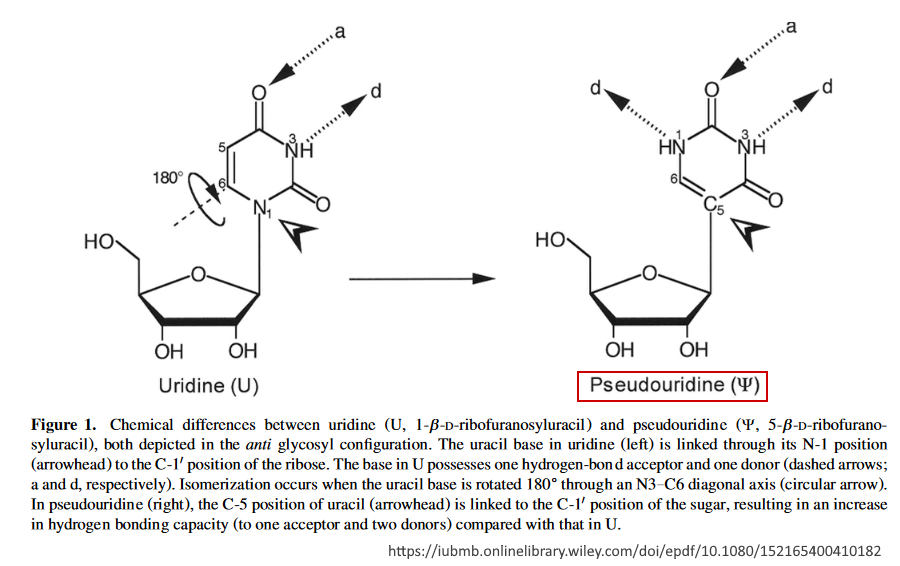
May 2000 – IUBMB Life: Pseudouridine in RNA: what, where, how, and why by Charette et al – READ, PDF
- “Pseudouridine (5-ribosyluracil) is a ubiquitous yet enigmatic constituent of structural RNAs (transfer, ribosomal, small nuclear, and small nucleolar). Although pseudouridine (psi) was the first modified nucleoside to be discovered in RNA, and is the most abundant, its biosynthesis and biological roles have remained poorly understood since its identification as a “fifth nucleoside” in RNA.”
- “Of the many intriguing features of RNA, none is more puzzling than the large number of structurally diverse residues (modified nucleosides) it contains…the first to be discovered, and the most abundant in RNA, is
pseudouridine (Y), the 5-ribosyl isomer of uridine (U). - Pseudouridine is synthesized at the polyribonucleotide level through the action of pseudouridine synthases, which catalyze the site-specifc isomerization of U residues in RNA.
1990
March 23, 1990 – Science: Direct Gene Transfer into Mouse Muscle in Vivo – Wolff, Malone et al – READ,
- “The seminal article of Wolf et al. shows that naked minimal nucleic acid vectors in the form of plasmid DNA (pDNA) or messenger RNA (mRNA) that code for a protein in an eukaryotic cell are spontaneously taken up and expressed in mouse muscles” – REF
1989
August 1, 1989 – PNAS: Cationic liposome-mediated RNA transfection – Robert Malone et al (The seminal paper) – READ – The history of mRNA vaccines by Jill Malone – READ, TIMELINE
1963
December 1, 1963 – Blood: Pseudouridine Metabolism. IV. Excretion of Pseudouridine and Other Nitrogenous Metabolites in Chronic Leukemia – Sherman Weissman et al – READ [Curious Surname, wondering if related to Drew Weissman?, Sherman participated in the Dec. 1984 meetings which in time launching the Human Genome project]