This page is intended to capturing data points for the key global COVID-19 vaccines from their conception to dates through clinical trials, approvals, rollouts, to boosters and beyond!
Focusing on Israel, UK, US, and Australia
Every time the governing bodies determine a booster dose is necessary the “vaccinated” become legally “unvaccinated” within a short time. So when the media and regulators claim the unvaccinated are filling the hospitals, how many injections have they received; none, two, three??
CDC carefully crafted their claim that the vaccines are “working very well” even though boosters are now required because of the new variant. There is no mention about preventing infected or preventing transmission of the virus. The reason to justify vaccination is about personal protection, to “prevent severe illness, hospitalization, and death”. Which is what they claimed these new vaccines could achieve from the start, completely different to what one would historically expect from a “vaccine“!
Preventing “disease” is different to preventing the virus from infecting or being transmitted. Dis-ease relates to symptoms. COVID-19 is the disease, SARS-CoV-2 is the virus that is said to cause the disease COVID-19. Is this why they are marketed as COVID-19 vaccines (preventing severe symptoms) rather than a SARS-CoV-2 vaccine (providing immunity)?
Without sterilising immunity against the virus, how is herd immunity via these “leaky” vaccines even remotely achievable?
The August 2021 new circulating variant (Delta) was the reason for the initial booster requirement, but the “vaccines” still encode the original Wuhan variant!
So if the original 2 shots (initial doses) of the Wuhan “spike code” allegedly protect you from the a Wuhan virus, how could how can a third or fourth dose of the Wuhan “spike code” provide additional protection from SARS-CoV-2 Delta variant that is shaped different. Is the risk:benefit ratio still the same?
Summary of Australia’s Provisional Registrations
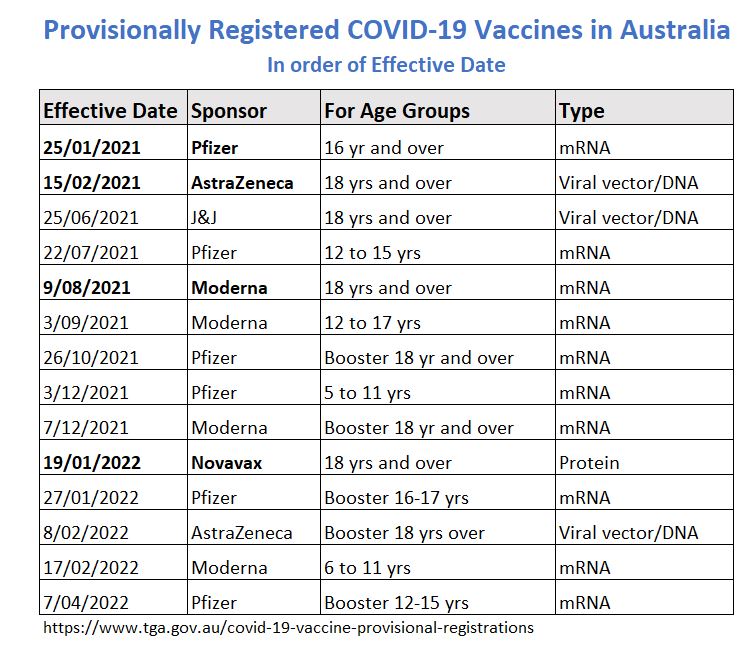
COVID-19 Vaccine Timeline
Data points below are automatically pulled from the greater COVID-19 timeline.
The below data points are specifically in reference to the COVID-19 vaccines.
Data points are continuously being added
Best viewed on a large screen!

Study: RNA shown to evade the innate immune system if altered to pseudouridine
DARPA awards Moderna $25M for mRNA development
On October 2, 2013 the US Defense Advanced Research Projects Agency (DARPA) through its “synthetic biology” ADEPT: PROTECT program, awarded Moderna Therapeutics “up to $25 million to research and develop its messenger RNA therapeutics™ platform as a rapid and reliable way to make antibody-producing drugs to protect against a wide range of known and unknown emerging infectious diseases and engineered biological threats.” Much of this funding will be used over the next 5 years to “advance promising antibody- producing drug candidates into preclinical testing and human clinical trials“. [1]
The ADEPT: PROTECT Pandemic Prevention Platform program plans to have the “development and widescale deployment of protective countermeasures” within 60 days from detection of an outbreak.
DARPAs’s Autonomous Diagnostics to Enable Prevention and Therapeutics: Prophylactic Options to Environmental and Contagious Threats – ADEPT: PROTECT program seeks to “enable adaptable, diagnostic devices that decrease the time required to design, manufacture, and rapidly distribute test panels in response to evolving or emerging diagnostic needs.”Moderna’s mRNA “platform has the potential to speed the development and manufacture of treatments that can produce a safer, more reliable and more robust immune response than existing technologies.”
The ADEPT program began in August 2011 when DARPA began investing in “Controlling Cellular Machinery (CCM) “technologies such as nucleic acid vaccines. The hypothesis was that rather than delivering antigens to the immune system, we could deliver genes that encode the antigen and allow the human body to produce the antigen from its own cells, triggering a protective immune response.”
In January 2014 DARPA created a new division called the Biological Technologies Office (BTO), “to explore the increasingly dynamic intersection of biology and the physical sciences” i.e. synthetic biology, neuro-nanotechnology and AI. [2, 3]
“DARPA pioneered the use of the body as a bioreactor to produce prophylactic antibodies to protect against biothreats”. With “[g]ene-encoded antibodies for near-immediate, temporary protection”. [4] This is different to gene-encoded antigen, which Moderna has claimed in 2020 to be a “vaccine”.
Time-Limited Provisional Registration added to TGA legislation
On March 29, 2018 the Australian government added a Provisional Registration (PR) amendment to the Therapeutic Goods Act (1989), of which the TGA Secretary now has overriding power to send a product application through the registration process with only preliminary clinical data. [3, 15]
On March 20, 2018 the TGA announce “As part of the Government’s response to the Review of Medicines and Medical Devices Regulation (MMDR review), we are implementing a pathway for the provisional registration of prescription medicines. Approval through the provisional pathway will be on the basis of preliminary clinical data where there is the potential for a substantial benefit to Australian patients. The provisional approval pathway allows sponsors to apply for time-limited provisional registration on the Australian Register of Therapeutic Goods (ARTG).”
This decision was publicised on June 26, 2017 as a “Priority review pathway for the registration of novel prescription medicines for Australian patients….[and] will involve faster assessment of vital and life-saving prescription medicines“. With specific eligibility criteria, of serious or life-threatening condition, where no existing alternative product is available.
The intent of this new registration category was explained in parliament in September 14, 2017 by the Health Minister, Mr Greg Hunt, as being intended as an avenue to provide “medicine” to those people with “significant unmet clinical needs for serious conditions”. [8, 9]
The process started October 24, 2014 with the announcement of the MMDR panel of 3 experts to review and report on the regulatory framework of therapeutic goods. [4, 5, 6, 7] The panel recommended a provisional pathway. [13, 14]
The government responded in 2016, noting the “international trends towards allowing earlier access to medicines” especially looking to fast-track “novel and life–saving medicines”, with a seeming emphasis on cancer drugs. [10]
- Provisional registrations are of a limited duration (max 2 years) and require the sponsor to supply ongoing clinical data to support their product.
- All PR products are part of the Black Triangle Scheme where healthcare professionals or consumers should report to the TGA all and any “any unfavourable and unintended sign, symptom or disease” following the use of the product “to help us build up the full picture of a medicine’s safety profile.” It’s not the doctor’s job to determine if a unintended consequence is related or not to the medicine.
- “For provisionally-registered medicines, the black triangle symbol will appear for a period of not less than five years.””
- Such “registrations” are added to the Australian Register of Therapeutic Goods (ARTG), but they are not classed as fully registered, they are still under assessment and may never receive full registration status.
Between 1 April 2018 until 22 June 2021 there has been [12] Provisional Registrations of which 2 are COVID-19 vaccines [1, 2] and 1 is remdesivir.
Immunisation Agenda 2030 – “leave no one behind”
In 2019 the World Health Organisation (WHO) began planning a new decade of vaccines called Immunisation Agenda 2030 (IA2030), superseding the Global Vaccine Action Plan (GVAP), that started in 2011 and expired 2020.
Vaccination or Immunisation, a practice the WHO claims saves “millions of lives every year”, is a component of 14 out of 17 of the United Nations Sustainable Development Goals (SDG). The plan is sold as “Immunization is an investment for the future, creating a healthier, safer and more prosperous world for all” and the intention is to “leave no one behind“, the market has opened up to everyone. [1, 2]
“Immunization is playing a critical role in achieving the Sustainable Development Goals (SDGs). Immunization reaches more people than any other health and social service, making it the foundation of primary health care systems and a key driver toward universal health coverage.” (Now think digital “vaccination certificate” – which will morph into passport)
In August 2020 at the 73rd World Health Assembly the new global vision for vaccines IA2030 was endorsed.
Johns Hopkins promotes Vaccine Platform Technologies
On April 23, 2019 the Johns Hopkins Center for Health Security publishes a report titled “Vaccine Platforms: State of the Field and Looming Challenges“. The project was sponsored by Facebook’s co-founder, Dustin Moskovitz’s “Open Philanthropy Project“, which coincidentally also sponsored the Event 201 coronavirus simulation. [1, 2, 3]
“[O]ver the past several years, [vaccine] platform technologies have been developed that could make it possible for multiple vaccines to be more rapidly produced from a single system.” In the report “the researchers describe major scientific and policy issues related to vaccine platforms” and “it provides recommendations aimed at helping realize the potential benefits of vaccine platform technologies” such as mRNA vaccines.
To date “there has been little in-depth analysis of platform vaccine technologies as a distinct class of technologies and approaches.” If these vaccine platform could be accepted by regulators and policy makers it would open the flood gates for investors and development, not to mention the promoted “urgent need for vaccines to combat emerging infectious disease outbreaks.”
Moderna/DARPA: First human Phase 1 trial using “mRNA therapeutic”
On September 12, 2019 Moderna announced study findings from their first ever human trial using a “mRNA therapeutic”, mRNA-1944, to encode the antibody protein, CHKV-24, for chikungunya “a mosquito-borne virus that poses a significant public health problem in tropical and subtropical regions”. [1, 2, 3, 4]
“These exciting data demonstrate a new way to address infectious diseases that uses mRNA to make antibodies in humans, establishing a powerful technology that could be deployable in a pandemic setting.”was commented in the press release. ‘[T]he results of this clinical trial validate that approach” DARPA representative stated.
Sponsored by US DARPA, the trial protocol was was first posted on Clinical Trials on February 4, 2019, taking 7 months to complete Phase 1 of the trial in 22 healthy adults, of which 6 received the placebo.
Phase 1 trial was “paused” in May 2020 due to the pandemic.
Novel coronavirus (2019-nCoV) genome sequence is made public
The sequence of the novel coronavirus (SARS-CoV-2) was posted to a public web server on January 10, 2020 [6]
Following China’s Jan 7, 2020 announcement a “viral genome sequence was released for immediate public health support via the community online resource virological.org on 10 January (Wuhan-Hu-1, GenBank accession number MN908947), followed by four other genomes deposited on 12 January in the viral sequence database curated by the Global Initiative on Sharing All Influenza Data (GISAID)”, according to Drosten et al. [4]
According to WHO on 12 January 2020, “China shared the genetic sequence of the novel coronavirus for countries to use in developing specific diagnostic kits.”
The genetic sequence of 2019–nCoV (now SARS-CoV-2), a new coronavirus associated with human respiratory disease in Wuhan, China (collection date 26/12/2019), was published on GISAID for countries to use in developing specific diagnostic kits. [3]
- The University of Sydney coordinated the notice of release of the genome to the world
- The complete genome sequence called SARS-CoV-2 isolate Wuhan-Hu-1
The virus is closely related genetically to SARS-CoV (82%) and to SARS-related bat and civet coronaviruses within the family Betacoronavirus, subgenus Sarbecovirus. [1, 2] The epidemiology of this subgenus is largely unknown, especially outside China.
China’s CDC report that on “January 3, 2020, the sequence of novel β-genus coronaviruses (2019-nCoV) was determined from specimens collected from patients in Wuhan by scientists of the National Institute of Viral Disease Control and Prevention (IVDC), and three distinct strains have been established.”
The genome sequence was published by China’s CDC. [5] The new Betacoronavirus genome sequence was deposited in GISAID (www.gisaid.org) under the accession numbers:
- EPI_ISL_402119
- EPI_ISL_402020
- EPI_ISL_402121
Moderna design mRNA vaccine sequence in one hour
Melissa J. Moore PhD, Chief Scientific Officer at Moderna recaps in a 2022 TED Talk that as soon as Chinese novel-CoV virus gene sequence was made public on January 10, 2020, they “got immediately to work”. [1, 2]
Within 2 days, following discussions with their National Institutes of Health (NIH) partners, they agreed on “which form of spike protein they’d put in their vaccines”. So on January 12, 2020 it “took Moderna’s mRNA design team just one hour to design” the modified mRNA vaccine sequence which was immediately put onto their manufacturing equipment and used in their phase 1 trials.
Remember, Moderna had never brought a product to market and the FDA considered mRNA as a “gene therapy“, yet by 2022 Moore refered to the use of their technology as a “vaccine” when used in a few trial patients who already had cancer (not as a preventative!).
“By January of 2020, we had already manufactured, quality controlled and delivered to several dozen patients personalised cancervaccines. So we had the know-how and the capacity to manufacture vaccines quickly.”
“There is a coming Tsunami of mRNA medicine” [called vaccines?]
US HHS-BARDA announce collaboration with Janssen/J&J on vaccine
On February 11, 2020, the US Health & Human Services (HHS), the parent body of the FDA, announced a collaboration with Janssen Research & Development, part of Johnson & Johnson, to help “develop coronavirus therapeutics, as well to “expedite development of vaccines that protect against the 2019 novel coronavirus”
The Biomedical Advanced Research and Development Authority (BARDA), headed by Rick Bright [1, 2, 3], is part of HHS & ASPR, they will collaborate with Janssen to identify compounds that have antiviral activity against SARS-CoV-2 as an initial step in developing new treatments.
BARDA will share research and development costs and expertise to help accelerate Janssen’s investigational novel coronavirus vaccine into clinical evaluation.
Caution warranted for coronavirus vaccines for humans – the animals died!
On February 27, 2020, independent media, The Highwire, alerted [@1:11:30] the public to the potential dangers of a SARS vaccine, base on a 2012 SARS vaccine study in mice. Coronovirus vaccines appear to cause “Pathogenic Priming”, a Disease Enhancement otherwise referred to as Antibody Dependent Enhancement (ADE). What resulted in the animal studies was upon re-infection the mice experienced a cytokine storm followed by death. [1, 2, 3]
The paper concluded “Caution in proceeding to application of a SARS-CoV vaccine in humans is indicated.”
- The same effect has been shown in chickens.
- In addition vasculitis or blood clots were identified in the coronavirus vaccine study in animals.
On March 5, 2020, Dr Peter Hotez warned the US government of “the unique potential safety problems of coronavirus vaccines”, such as with RSV vaccines in the 1960’s where “paradoxical immune enhancement phenomenon” can occur with respiratory virus vaccines, and “we don’t entirely understand the basis of it”! They were confronted with the same “immune pathology” problem with coronavirus vaccine tests done on laboratory animals. The FDA are aware of the problem, these types of vaccines can’t be rushed because of the long-term safety implications. [@1:26:30]
Yet Operation Warp Speed was officially announced 2 months later, .
WHO: Solidarity Vaccine Trial initiative begins
On April 9, 2020 WHO released their Solidarity Vaccine Trial protocol an ” international randomised trial of candidate vaccines against COVID-19″, those candidates that meet the WHO’s criteria. To coordinate the evaluation of more than 70 vaccines which are currently in development, and 3 in clinical trial as of April 11, 2020.
This WHO vaccine acceleration initiative is headed by Andrew Witty, former CEO of GlaxoSmithKline. [2, 3]
It is unknown what will happen to global vaccine production and supply if the WHO prioritise the same vaccine candidates as the US Warp Speed initiative! [1]
Bill Gates: a vaccine for 7 billion people is the only solution
Self proclaimed health expert, Bill Gates, who has no medical or scientific qualifications, but is a “global health” philanthropist, was interviewed [with seemingly scripted questions] on BBC Breakfast on April 12, 2020 and told us that:
“the thing that will get us back to the world that we had before coronavirus is the vaccine and getting that out to all 7 billion people”
There was no vaccine at this time, they were all experimental and in early trials. And why did all 7 billon need a shot when many people already had been natural infected and mounted an immune response? Could it be because he had investments in the vaccine companies, and would make a fortune if they were force upon the world?
Bill also claimed that “we didn’t simulate this, we didn’t practice” yet in October 2019 his foundation was a co-sponsor of a coronavirus pandemic simulation called Event 201!
Bill is pushing for “global cooperation” and more funding. According to Bill the “rich” countries are now experiencing the “second wave” of “very challenging epidemics”, though he predicted the “developing countries who yet don’t have a large number of cases” are likely to be worst hit because “their ability to isolate” and their health systems are far less than the rich countries. “So the global cooperation is to help those counties“. [1]
Other quotes by Bill:
“And of course the vaccine is a protective, to prevent you from getting sick”
“…so we’re going to have to take something that usually takes 5 to 6 years [to develop] and get it done in 18 months. There is an approach called an RNA vaccine…that looks quite promising…unfortunately the schedule for the [conventional vaccine approach] will probably not be as quick as the RNA platform, that we’ve been funding directly and through CEPI over the last decade.”
“…this is such an unprecedented, very tough thing to deal with. The people like myself and Tony Fauci are saying 18 months [to develop a vaccine], if everything went perfectly we can do slightly better than that, but there will be a trade off, we’ll have less safety testing than we typically would have, and so governments would have to decide do they indemnify the companies… we just don’t have the time to do what we would normally do“
Safety testing: Gates compares rushed drug approval for HIV [someone who is sick or tests positive] to the safety trade-off of fast vaccine development given to healthy people! “…this is a public good, so those [safety] trade-offs…the regulator says go ahead even though you haven’t taken the normal time period”
“I do think now, because this has been so dramatic, ahhh, we weren’t ready for this pandemic but I do think we will be ready for the next pandemic, and using the new tools of science [mRNA platform?] that’s very very doable.“
The host prompts “…are you optimistic that now…there will be a different mindset around the fears around viruses and pandemics”
“…we should be able to have a vaccine in less than a year if we’re on standby with the right factories and the right science…”
“…a big missing piece is funding the research for these type of vaccines…[jumble!]”
“its shocking…how hard its going to be to get back to normal life that we had before”
[Do you think things will go back to normal?] “Once you have a safe and effective vaccine and get that out to all most all of the people on the planet, and build the preparatory systems for the next pandemic…we will go back to normal and economies will recover…innovation will help us not be at such a risk in the years after that.“
Its worth watching the 17 minute INTERVIEW which the “Trusted” BBC have “unlisted” on YouTube.
BioNTech begin Phase I/II human trails
On 23 April 2020 the first 12 study subjects are vaccinated with the BioNTech mRNA vaccine candidate after Germany regulator approves Phase I/II clinical trial. [1]
Oxford Uni begin Phase I vaccine clinical trial
The first two volunteers, one control (meningitis vaccine) and the other the vaccine treatment, were injected on April 23, 2020 for the Oxford University Phase I human vaccine clinical trials. [1]
The Oxford researchers started screening healthy volunteers (aged 18-55) in March 2020 for their upcoming ChAdOx1 nCoV-19 vaccine trial in the Thames Valley Region.
Seven days later on April 30, 2020 Oxford University announces partnership with AstraZeneca to help develop and distribute their COVID-19 vaccine.
Spike (S) protein has 14 mutations identified – consequential for vaccines
On April 30, 2020 a pre-print paper by Korber et al, (now peer reviewed) had identified 14 mutations in the Spike (S) protein of the SARS-CoV-2 virus. [1, 2]
This is highly consequential as the Spike protein “mediates infection of human cells and is the target of most vaccine strategies and antibody-based therapeutics.” Mutations in this region of the virus “may confer selective advantages in transmission or resistance to interventions”
It was noted that this mutation of “Spike D614G is of urgent concern” They first identified G614 mutation in Italy sample on February 20, 2020 where it “began spreading in Europe” and found “when introduced to new regions it rapidly becomes the dominant form” indicating highly transmissible.
Noteworthy also is they found “evidence of recombination between locally circulating strains” in the S943P mutation, meaning an infected person is infected with multiple virus strains, not just one, and the stains can re-combine their genetic material to form new “recombinant” virus strains.
Oxford monkey study fails to stop viral infection or transmission – but proceed to human trials
On May 13, 2020 Oxford and NIAID scientists published the pre-clinical animal study looking at vaccinated versus un-vaccinated rhesus macaques (monkeys). They “observed a significantly reduced viral load in bronchoalveolar lavage fluid and respiratory tract tissue of vaccinated animals challenged with SARS-CoV-2 compared with control animals, and no pneumonia was observed in vaccinated rhesus macaques.” [1]
But the Oxford/AstraZeneca vaccine in this animal challenge study, “did not provide sterilizing immunity” which is considered the “gold standard for any vaccine.” Vaccinated monkeys could still become infected and had viable virus in their nose which could be transmitted and infect others!
Even though “no evidence of immune-enhanced disease following viral challenge in vaccinated animals was observed”, experience with other vaccines tells us that is not a firm guarantee that such will be the case for humans, states William Haseltine
Based on the observation that their vaccine could “moderate the disease” they proceeded into human clinical trials.
“Operation Warp Speed” announced
On May 15, 2020, following March 2, 2020 discussions with pharmaceutical executives, President Trump announced a U.S. public-private partnership, the Operation Warp Speed Vaccine Initiative (OWSVI), to accelerate the development, manufacture, and distribution of COVID-19 vaccines, therapeutics, and diagnostics collectively known as countermeasures. With the aim of delivering 300 million doses of a safe, effective vaccine for the entire population of the United States with an effective vaccine “before the end of the year.” [1, 2, 3, 4, 5, 6]
“President Trump’s vision for a vaccine by January 2021 will be one of the greatest scientific and humanitarian accomplishments in history, and this is the team that can get it done,” said HHS Secretary Alex Azar.
OWS is headed by ex GSK’s & ex Moderna’s director Dr Moncef Slaoui, [7] with projects led by:
- Vaccines: Peter Marks, M.D., Ph.D., Director of the FDA’s Center for Biologics Evaluation and Research.
- Therapeutics: Janet Woodcock, M.D., Director of the FDA’s Center for Drug Evaluation and Research.
- Diagnostics: Bruce Tromberg, Ph.D., Director of the NIH’s National Institute of Biomedical Imaging and Bioengineering.
At this point Dr Anthony Fauci is not confident a vaccine will be effective. A warp speed vaccine could be deadly.
Eleven days earlier the EU hosted a global pledging event where the US abstained.
The statistics are that only 1 in 15 vaccines that enter phase II trials is ever licensed, and the average development time for vaccines is usually measured in decades. [2] On June 5, 2020, OWSVI had chosen 5 vaccine candidates: Moderna, Oxford/AstraZeneca, Johnson & Johnson, Merck and Pfizer, the first three having already received $2.2 billion in federal funding.
Pfizer chose not to participate in the OWS government ran, DOD program (though they did have a contract), as a consequence, in 2024 they left themselves open to being sued by US States – EXCERPT
Uni Oxford begin Phase II/III vaccine clinical trial
University of Oxford researchers have begun recruiting for the next phase in human trials of a COVID-19 vaccine 10,260 adults and children at partner institutions across the country.
Adult participants in both the Phase II and Phase III groups will be randomised to receive one or two doses of either the ChAdOx1 nCoV-19 vaccine or a licensed vaccine (MenACWY) that will be used as a ‘control’ for comparison.
COVID-19 vaccine developers have a problem – not enough sick people
As early as May 22, 2020 the lack of infectious cases of COVID-19 was identified by Adrien Hill from Oxford’s Jenner institute when he said to Science mag “…we’re beginning to run out of good trial sites to do vaccine efficacy studies—even the U.S. is plateauing,” …People are going to fight for that site to get the vaccine tested before it runs out.” The disappearance of Ebola cases in November 2015 was a major problem for vaccine developers!
Then on June 10, 2020 the Washington Post reported that Oxford University officials who were rushing to “develop coronavirus vaccines are alerting governments, health officials and shareholders” that declining numbers of new infections may be getting too small to quickly determine whether vaccines work!
“Even as new cases are growing worldwide, transmission rates are falling in Britain, China and many of the hardest-hit regions in the United States — the three countries that have experimental vaccines ready to move into large-scale human testing in June, July and August.”
Volunteers need to be exposed to someone infected with the virus to determine if the vaccine works.
Fauci: coronavirus vaccines may not provide long-term immunity
When talking with JAMA Editor Howard Bauchner, Dr. Anthony Fauci says there’s a chance the coronavirus vaccines may not provide long-term immunity, if COVID-19 acts like other coronaviruses, “it likely isn’t going to be a long duration of immunity”. [1]
At this point “scientists still don’t fully understand key aspects of the virus, including how immune systems respond once a person is exposed.”
At the same time member of the boards of Pfizer and former FDA commissioner, Dr Scott Gottlieb says expect COVID-19 vaccine to be seasonal like the flu shot.
Gavi COVAX AMC 92 set up the financial arm to finance COVID-19 vaccines for developing nations
At the June 4, 2020 at the virtual Global Vaccine Summit pledging event, Gavi launched the Gavi COVAX AMC as an “investment opportunity” and first building block of the COVAX Facility. A required “financial mechanism” so that the world’s poorest countries will get access to COVID-19 vaccines. It required $2 billion to starting investment. [1]
Gavi coordinated and raised funds for the COVAX AMC, an innovative financing instrument that supported the participation of 92 lower-income economies in the COVAX Facility.
“The goal is by the end of 2021 to deliver two billion doses of safe, effective vaccines to all participating countries including the 92 AMC-eligible economies. Once a vaccine has been approved by regulatory agencies and/or prequalified by the WHO, the COVAX Facility will then purchase these vaccines with a goal to try and initially provide doses for an average of 20% of each country’s population, focusing on health care workers and the most vulnerable groups.”
- The COVAX facility documents were released August 2020
- On top of 2020 funding, more pledges were made to COVAX initiative at 2021 G7 summit
- COVAX AMC 92 framework
- Gavi plan to push COVID-19 vaccines into 2024-25
- By 2024 Gavi has raised more than US$12 billion in donor funding for the AMC
Warp Speed chooses 5 vaccine candidates
On June 5, 2020, the Operation Warp Speed (OWSVI) had chosen 5 vaccine candidates: Moderna, Oxford/AstraZeneca, Johnson & Johnson, Merck and Pfizer, the first three having already received $2.2 billion in federal funding. [1, 2]
Former FDA chief Scott Gottlieb, and Pfizer board member, pointed out Sanofi and Novavax were absent from the list. Paul Offit also questions “the lack of diversity in the five selected vaccines, which rely on just three different technologies.” An unnamed source connected to the selection process said “It’s been so chaotic, and it’s not even transparent to those of us who are trying to help out.”
“The move appeared to signal that Warp Speed had changed its initial plan of doing comparative studies of 14 vaccines it said last month that it had singled out from the more than 100 candidates in development at companies and universities.”
FDA sets a low efficacy requirement of 50% for COVID-19 vaccines, WHO sets 70%
In a press release on June 30, 2020 the US FDA issued guidance to manufacturers “help facilitate the timely development of safe and effective vaccines to prevent COVID-19” in order to win regulatory approval. [7]
“The guidance also discusses the importance of ensuring that the sizes of clinical trials are large enough to demonstrate the safety and effectiveness of a vaccine. It conveys that the FDA would expect that a COVID-19 vaccine would prevent disease or decrease its severity in at least 50% of people who are vaccinated.” Also the vaccine companies would be required to monitor the vaccine’s performance after approval for any emerging safety problems. [1, 2, 3]
“If you had a 60 or 70 percent effective vaccine and everybody took it, you might actually be reaching toward herd immunity and potentially then dampen down this pandemic,” Dr George Poland of the Mayo Clinic said on Nov 3, 2020.
As a comparison the flu vaccine effectiveness which “can vary widely from year to year has been anywhere from 20% to 60% effective over the last decade.
According to GlobalData on April 9, 2020 the WHO sets two vaccine success benchmarks for vaccines. “Preferably, the vaccine should have at least a 70% efficacy on a population basis with durability for at least a year for reactive use in an outbreak and/or protection for those with a high ongoing risk. The lower success bar is about 50% efficacy with at least a six-month durability”. [4]
They go on to state: “The 50% success bar, while low, is acceptable, as it would likely be enough to ease the pressure on frontline healthcare resources but it may not be high enough to reach herd immunity.”[6]
FDA commissioner Dr Stephen Hahn in a JAMA interview on July 30, 2020, stated “We all want a vaccine tomorrow. That’s unrealistic. And we all want a vaccine that’s 100% effective. Again, unrealistic,” Hahn said “we said 50%, and the reason was because we felt that that was a reasonable floor given the pandemic.” [5]
A couple of days earlier, Dr Anthony Fauci said he would like 60% meaning on average the vaccine reduces a person’s risk of SARS-CoV-2 infection by 60%.
ICAN demands FDA mandate inert placebo controls in COVID-19 vaccine clinical trials
Upon finding out AstraZeneca COVID-19 Vaccine clinical trials were not using a placebo control, but a neningococcal vaccine (Menveo) as a “control”, the Informed Consent Action Network (ICAN) a non-profit organisation, petitioned the FDA to mandate inert placebo control groups in US COVID-19 vaccine clinical trials, as well as track the safety for the long-term in properly sized trial groups. [1, 2]
Nine days after ICAN filed its initial petition, on June 30, 2020, the FDA changed course and issued emergency guidance to industry that all COVID-19 clinical trials must use a placebo control.
Later ICAN sued the FDA to supply the safety studies on the Menveo vaccine, in case they intended to approve it as an active control for US EUA products.
Many don’t realise using another vaccine for the “control” group to assess “vaccine safety”, is a common and accepted practice.
They don’t use inert placebo controls. This is why post marketing surveillance is so important, but the authorities have failed in their duty.
FDA reveal EUA criteria – “No adequate, approved, and available alternative”
Dr. Doran Fink from the FDA explains at the July 29, 2020 ACIP meeting the criteria necessary for the FDA to be able to issue a COVID-19 vaccine with Emergency Use Authorisation (EUA).
The key point being that there has to be “No adequate, approved, and available alternative“. [@2:57]
COVID-19 vaccine global access (COVAX) facility – to accelerated vaccine development and manufacture
On August 6, 2020 WHO released their COVID-19 vaccine global access (COVAX) facility document to justify their “accelerated vaccine development and manufacture.” [1]
 WHO, Gavi and CEPI launched the COVAX facility to ensure global equitable access to COVID-19 vaccines and end the acute phase of the pandemic by the end of 2021. [9]
WHO, Gavi and CEPI launched the COVAX facility to ensure global equitable access to COVID-19 vaccines and end the acute phase of the pandemic by the end of 2021. [9]
“Developing a vaccine against COVID-19 is the most pressing challenge of our time” claiming the IMF estimated in April 14, 2020, that a vaccine [that didn’t exist] would “prevent the loss $375 Billion to the global economy every month”.
COVAX is the “vaccine” arm of the April 2020 United Nations collaborative ACT Accelerator
- Gavi launched the finance arm, COVAX AMC on June 4, 2020
- Australia’s Jane Halton, who is the Chair of CEPI is also the co-chair of COVAX. Australia has signed agreements. “In early 2020, CEPI raised US$2b to expand the number of vaccine candidates to increase the chances of success, and fund the clinical trials”.
- The COVAX Shareholders Council meetings began November 3, 2020 in Geneva. [10]
- COVAX coorinated vaccine roll out to to 92 low- and middle-income economies.
- COVAX began to take shape early 2020, and was sold based on the claim that “no one is safe, unless everyone is safe”, where “vaccines” are that saviour. [2]
- COVAX was led by GAVI [4, 5], CEPI and the WHO, alongside key delivery partner UNICEF
- Feb 22, 2023 – On behalf of COVAX partners WHO developed a No-Fault Compensation Program – the first and only global vaccine injury compensation mechanism
- Jan 31, 2021 the WHO “had to” generate a “tool” to encourage “acceptance and demand” for COVID-19 vaccines! as part of the Country Readiness and Delivery (CRD) workstream [3, 7, 8]
- January 2022 the COVID-19 Vaccine Delivery Partnership (CoVDP) was launched
- COVAX closed December 31, 2023 [6]
AstraZeneca Phase III trial halted over suspected serious vaccine reaction
On September 8, 2020, Oxford/AstraZeneca (A/Z) “voluntarily” halted their COVID-19 vaccine Phase III clinical trial after a previously health, UK trial participant, came down with an unexplained suspected vaccine reaction. [1]
The following day A/Z CEO announced the female trial participant had experienced neurological symptoms consistent with a rare but serious spinal inflammatory disorder called transverse myelitis, that can cause muscle weakness, paralysis, pain and bladder problems.
It was only a week earlier, on August 31, 2020 that the US began the AstraZeneca Phase III trials. [2]
On October 26, 2020 AstraZeneca resumed their US Phase III trials after an “independent safety board reviewed the incident and determined the participant’s illness was unrelated to AstraZeneca’s coronavirus vaccine candidate”, though in the UK they resumed earlier on September 15th.
UK’s MHRA expects “high volume” of CV-19 vaccine side effects
On September 14, 2020 UK Medicines & Healthcare products Regulatory Agency (MHRA) via their MHRA Buyer Organisation awarded a 1.5 million GBP contract to Genpact (UK) Ltd to “urgently” develop a “an Artificial Intelligence (AI) software tool to process the expected high volume of Covid-19 vaccine Adverse Drug Reaction (ADRs) and ensure that no details from the ADRs’ reaction text are missed.” [1, 2, 3, 4]
The “Contract award notice” which was published October 19, 2020 on Tenders Electronic Daily (TED) stated:
For reasons of extreme urgency under Regulation 32(2)(c) related to the release of a Covid-19 vaccine MHRA have accelerated the sourcing and implementation of a vaccine specific AI tool. …[As] it is not possible to retrofit the MHRA’s legacy systems to handle the volume of ADRs that will be generated by a Covid-19 vaccine.
The contract was awarded “without prior publication“, suggesting MHRA had Genpact in mind to begin with? [5]
CDC to use V-SAFE, a new app system to actively monitor COVID-19 vaccines outside clinical trial setting
On September 22, 2020 the CDC’s COVID-19 Vaccine Planning Unit (VPU) presented their plan, once the vaccines rolled out, to conduct active “enhanced safety monitoring for COVID-19 vaccines in early phase vaccination” through smartphone and email-based web surveys, in addition to VAERS.
The CDC introduced the Vaccine Safety Assessment For Essential workers (V-SAFE) phone app at vaccine roll-out, initially intended for health workers, though was made available to the general public to download and register. For the first week participants would get prompted to enter post vaccination information, then weekly up to [only] 6 weeks – this is called active surveillance. [2]
The purpose of the V-SAFE app “is to rapidly characterize the safety profile of COVID-19 vaccines when given outside a clinical trial setting.” Data submitted to V-SAFE is “collected, managed, and housed on a secure server by Oracle,” a private, third-party computer technology company who receives the data in deidentified form. [3]
The US Vaccine Adverse Events Reporting System (VAERS) is “the US early warning safety monitoring system”, co-managed by the CDC and FDA, with an established baseline yearly reporting rate. [1] The management of vaccine safety has historical issues!
By December 2021 the CDC refuse, through FOIA, to release the deidentified V-SAFE data to the public which Oracle is allowed to manage!
WHO changes the definition of “Herd Immunity”
The WHO starts steering the definition of ‘herd immunity’ to be attributed ONLY to vaccinated populations and removing the [known] reference to immunity gained from natural infection of a pathogen. The WHO web page first archived this change on October 15, 2020. [2]
Then by December 31, 2020 the WHO updates the herd immunity definition to again include “natural infection“.
They also removed the reference to antibodies. This is important as binding antibodies vs neutralising makes a big difference, especially for coronavirus vaccines, where immune enhancement has been an issue in previous animal studies.
Herd immunity is a known concept associated of disease spread in a population, such that if one person has the infection, they spread it to one or less other people, as Professor Bhattacharya explains.
Watch >>>
WHO introduces a new concept: “Vaccines are Immunity”
WHO’s Strategic Advisory Group of Experts (SAGE) on immunization release a document [v1, v1.1] that starts to introduce the idea that “vaccines are immunity“, ignoring the science of natural immunity.
The World Bank: promotes the End of the pandemic March 2022 by reaching herd immunity through vaccinating 60% global population.
COVID-19 vaccine clinical trial protocols
The full clinical trial protocols for the COVID-19 vaccines for which ICAN filed its petitions were released to the public.
See copies for manufacturer’s vaccines:
Those protocols revealed that some of ICAN’s demands regarding the duration for tracking vaccine safety had been met.
Pfizer vaccine batch integrity issues known to regulators
From leaked emails and reports to Trial Site News in June 2022, it indicates that as early as November 10, 2020, the European regulatory agency (EMA) staff, who oversees the evaluation of medicinal products for the European Union, had reason to be concerned about the rushed speed of the regulatory process with respect to robustness of assessment, plus they were aware of potential issues with Pfizer-BioNTech’s vaccine batch integrity.
The emails reveal that regulatory bodies like the FDA, MHRA, EMA and Health Canada knew of the differences in batches, regarding % mRNA integrity and the presence of uncharacterised fragments of RNA in batches (“impurities”), making the ‘safety and efficacy’ of the COVID-19 vaccine an unknown and could account for the variation in adverse reactions to batch/lot numbers.
Knowing these issues the regulators granted various designations of emergency use authorisation a few weeks to months later – which would not pass normal regulation.
Moderna: COVID-19 vaccine has 94.5% efficacy
In a press release on November 16, 2020, Moderna announced their intended to submission to the FDA for Emergency Use Authorisation of their COVID-19 vaccine, on the back of their Phase 3 trial returning 94.5% efficacy. [1]
A fuller analysis was released on November 30, 2020, which showed a 94.1% efficacy.
Pfizer-BioNTech announce their vaccine is 95% effective
In a press release on November 9, 2020, Pfizer-BioNTech announce their preliminary phase 3 clinical trial data suggests their COVID-19 vaccine is more than 90% effective. [1, 2]
Then they announced via press release on November 18, 2020, that the final analysis of their COVID-19 vaccine Phase III clinical trial data showed it was 95% effective and with no safety concerns, stating:
“Primary efficacy analysis demonstrates BNT162b2 to be 95% effective against COVID-19 beginning 28 days after the first dose;170 confirmed cases of COVID-19 were evaluated, with 162 observed in the placebo group versus 8 in the vaccine group”.
“Efficacy was consistent across age, race and ethnicity demographics. The observed efficacy in adults over 65 years of age was over 94%,” [1]
Those results had yet to be peer reviewed, but the media ran with the claim. Pfizer filed for emergency use authorization with the FDA “within days” of this announcement.
CDC publish baseline mRNA COVID-19 vaccine information – vaccine stays in the arm muscle, and spike is “harmless”
On November 23, 2020 the US CDC published information on the expected to be authorised new technology mRNA COVID-19 vaccines. [1, 2]
Stating “facts” that emerging science showed, turned out to be misinformation.
- The “immune response” that “produces antibodies, is what protects us from getting infected if the real virus enters our bodies”.
- The protein which the COVID-19 mRNA vaccines instructs the human cells to make was advertised as “a harmless piece of what is called the “spike protein.””
- The “vaccines are given in the upper arm muscle. Once the instructions (mRNA) are inside the muscle cells, the cells use them to make the protein piece. After the protein piece is made, the cell breaks down the instructions and gets rid of them.” Which the media then spread the world, quoting CDC. [5] – It was soon discovered the LNP carrying mRNA dosnt stay in the arm.
- “At the end of the process, our bodies have learned how to protect against future infection. The benefit of mRNA vaccines, like all vaccines, is those vaccinated gain this protection without ever having to risk the serious consequences of getting sick with COVID-19.”
- “mRNA never enters the nucleus of the cell, which is where our DNA (genetic material) is kept.” – (No evidence to support this statement) [3]
- “mRNA COVID-19 vaccines cannot give someone COVID-19″ – [COVID-19 is a collection of symptoms, the SARS-CoV-2 spike protein is demonstrating to cause COVID-19 like symptoms]
The only COVID-19 vaccines theFDA will grant emergency use authorization are those that meet the ” rigorous safety and effectiveness standards” guidelines, of amongst other things, >50% with placebo controlled trials.
Researchers have been studying and working with mRNA vaccines “for decades” even though there “are currently no licensed mRNA vaccines”.
“Interest has grown in these vaccines because they can be developed in a laboratory using readily available materials. This means the process can be standardized and scaled up, making [ALL] vaccine development faster than traditional methods of making vaccines.”
“Future mRNA vaccine technology may allow for one vaccine to provide protection for multiple diseases, thus decreasing the number of shots needed for protection against common vaccine-preventable diseases.”
The CDC later quitely removed from website, their claim that the “mRNA and the spike protein do not last long in the body”, which follows is later edited after the science shows it can remain in blood for at least 28 days, let alone the arm muscle.
TGA assure rigorous assessment before COVID-19 vaccines approved
On November 27, 2020 Australia’s Therapeutics Goods Administration (TGA) state on their website that they “will rigorously assess any COVID-19 vaccine for safety, quality and effectiveness before it can be supplied in Australia.”
On March 29, 2018 the Therapeutics Goods Act 1989 was amended to include a Provisional Registration clause to allow products to enter the system on limited data, not allowed for normal registration. The clause was sold on the intent of providing life-saving medication to cancer patinets, and to have our regulatory system was in line with the rest of the world – with a fast-track, emergency-like provision!
Pfizer documents show they knew their vaccine had limited efficacy
For first 3 months post FDA authorization Pfizer received multiple reports of both vaccine failure and vaccine ineffectiveness according to FOIA documents.
Pfizer’s internal documents that were released by court order in 2022 under FOIA revealed that beginning on December 1, 2020, Pfizer was aware by February 28, 2021 that their vaccine had limited efficacy and that 136 people died following injection!
UK the first country to authorise a COVID-19 vaccine
On December 2, 2020, the Pfizer-BioNTech COVID-19 vaccine was “temporarily authorised“, by UK’s Medicines and Healthcare products Regulatory Agency ( MHRA), becoming the first COVID-19 vaccine to be authorised anywhere in the world, “paving the way for mass vaccination”. [1, 2]
The vaccine was reported to offer “up to 95% protection against Covid-19 illness”. Pfizer’s CEO stated on December 3, 2020 that Pfizer don’t know if the vaccine will prevent transmission – the entire purpose of a mass vaccination campaign.
The governments authorisation “follows months of rigorous clinical trials and a thorough analysis of the data by experts at the MHRA who have concluded that the vaccine has met its strict standards of safety, quality and effectiveness”, contradictory to Pfizer FOIA documents.
On 30 December “the cheaper and easier-to-distribute Oxford-AstraZeneca COVID-19 vaccine” was approved. A third vaccine, produced by Moderna, was approved for use in the UK in January 2021. Finally, Janssen’s single-dose vaccine was approved in May 2021, although it is yet to be used. [1, 2]
US COVID-19 vaccination cards released
The US Department of Defense (DoD) released the first images of a COVID-19 vaccination record card and vaccination kits on December 3, 2020, prior to anticipated EUA of COVID-19 vaccines. [3]
“Vaccination cards will be used as the “simplest” way to keep track of Covid-19 shots, said Dr. Kelly Moore, associate director of the Immunization Action Coalition, which is supporting frontline workers who will administer Covid-19 vaccinations.” [1, 2]
The 100 million DoD vaccine kits “include a card, a needle and syringe, alcohol wipes and a mask. Operation Wa
The card for the 2-shot vaccines already has additional spaces for 2 booster shots – as though it was planned! The vaccination clinics are to also report the data electronically to their state immunization registries and the CDC – making a card redunant unless they planned ahead of time to use it as “proof of vaccination” to be eligible to move in society
Pfizer CEO ‘not certain’ if their vaccine will prevent transmission
On December 3, 2020, just one day after the UK became the first country to grant emergency use authorisation for Pfizer-BioNTech’s COVID-19 vaccine, it was reported that Pfizer’s CEO Albert Bourla said in an interview with NBC that the company is “not certain” if their vaccine will prevent transmission, “I think this is something that needs to be examined” he said. [1, 2]
First COVID-19 vaccine administered in UK, which is the first western country to rollout the vaccine
As planned, on 7 December, 2020 the UK is the first western country to roll out it’s emergency use COVID-19 vaccine, of which 90 year old Margaret Keenan publicly received the first dose of Pfizer-BioNTech COVID-19 vaccine (she gets second dose on Dec 30) and William Shakespeare was the first man to receive the vaccine outside of clinical trials. [1]
“On 2 December 2020, the Pfizer-BioNTech Covid-19 vaccine was approved for use in the UK, becoming the first to be authorised anywhere in the world. This was followed on 30 December by the cheaper and easier-to-distribute Oxford-AstraZeneca vaccine.” [2]
The US administered their first COVID-19 vaccine on December 14, 2020
Hacked EMA data reveals potential significant CV19 mRNA batch integrity issues
On December 9, 2020 the European Medical Agency (EMA) was the subject of a cyberattack which was revealed that “COVID-19 medicines and vaccines” [1] data was stolen. This included “email screenshots, EMA peer review comments, Word documents, PDFs, and PowerPoint presentations”, of which some of those documents related to “the regulatory submission for Pfizer and BioNTech’s COVID-19 vaccine candidate, BNT162b2.” [2]
Nineteen days after the hack, on December 21, 2020, the EMA granted Conditional Marketing Authorization (CMA) to Pfizer-BioNTech, for the very vaccine in question – which the hack reveals that the EMA regulators had at the time over 100 regulatory objections.
On of the biggest objection was reported by a BMJ investigation published on March 10, 2021, that revealed by November 23, 2020 the EMA “regulators had major concerns over unexpectedly low quantities of intact mRNA” …”between the clinical batches and proposed commercial batches—from around 78% to 55%.” Revealing concerning batch integrity instability. [3, 4, 5, 6]
Australia cancels 51 million doses of Uni QLD vaccine due to HIV scare
Australian government drops Uni Qld, CSL, CEPI COVD-19 vaccine after volunteers in early clinical trials “falsely” tested positive for HIV. A pre-order for 51 million vaccine doses was cancelled. $750 million investment. Trials began on July 13, 2020 with the involvement of CSIRO.
Health Minister Greg Hunt said that while the HIV test results were false, “the scientific advice is that the risk to vaccine confidence was the principal issue here.” [1]
This vaccine generates antibodies for the “Molecluar Clamp” that is “critical for driving membrane fusion and cell entry”. The HIV-1 coat protein – GP41 is known to be neurotoxic. [2]
FDA issues first COVID-19 vaccine EUA for Pfizer-BioNTech with no data on “preventing transmission”
On December 11, 2020, the US Food and Drug Administration (FDA) granted the first Emergency Use Authorisation (EUA) for a brand new technology product, the Pfizer-BioNTech COVID-19 mRNA vaccine, just 9 months after phase I trials began, and after only 108 days of regulatory safety review. In their press release they stated: “The FDA has determined that Pfizer-BioNTech COVID-19 Vaccine has met the statutory criteria for issuance of an EUA”. The FDA stated the vaccine “may be effective in preventing COVID-19″, then state it is 95% effective in “preventing” COVID-19, that 95% is based on “a 2 month study of a couple hundred people. That’s it!“. [3]
The day before, on December 10, 2020, the Vaccines and Related Biological Products Advisory Committee (VRBPAC) met to assess the Pfizer-BioNTech COVID-19 Vaccine and prepare a Briefing Document for the FDA. [6]
The FDA stated “[t]he vaccine was 95% effective in preventing COVID-19 disease among these clinical trial participants with eight COVID-19 cases in the vaccine group and 162 in the placebo group.” 8 plus 162 equals 170 trial participants was all that were used to determined the relative risk of “95% effective”, where as the absolute risk (of all 36,523 trial participants) works out to less than 1% effective. [4]
“At this time, data are not available to make a determination about how long the vaccine will provide protection, nor is there evidence that the vaccine prevents transmission of SARS-CoV-2 from person to person.”
President Trump announced this as a “medical miracle” and vaccines would be available within 24 hours. [1, 2]
The rush to market was the result of President Trumps‘ Operation Warp Speed, where he allocated “$14 billion to accelerate vaccine development and to manufacture all of the top candidates in advance”.
- 11 Dec 2020 – EUA* for Pfizer CV19 vax – 30ug/dose
- 18 Dec 2020 – EUA for Moderna CV19 vax [5] – 100ug/dose
*EUA for an unapproved product
Under EUA these new products referred to as ‘vaccines’ are still undergoing data collection and thus are experimental. American frontline doctors have raised many concerns in a white paper, which includes:
- No vaccine based on messenger RNA (mRNA) has ever been approved for any disease, or even entered final-stage trials until now, so there’s no peer-reviewed published human data to compare how mRNA stacks up against older technologies.
- Previous coronavirus vaccine projects triggered re-challenge immune responses so strong that the test animals died, and the vaccine trials were halted. Scientists have never been able to create a successful coronavirus vaccine.
Is this new technology a “vaccine” or “medical device“?
The vaccines start rolling out across the US on Monday December 14, 2020, when early treatments are still suppressed.
Pfizer unblinds placebo control arm of trial group
On December 14, 2020 Pfizer/BioNTech removes the saline placebo control arm (unblinds) of their Phase 3 clinical trial, just 3 days after FDA issues Emergency Use Authorisation (EUA) for Pfizer-BioNTech COVID-19 vaccine. This unblinding of the control group by offering the trial participants the option to take the treatment product (the vaccine) eliminates the true ability to conduct legitimate long term safety studies.
- Dec 14, 2020 – Pfizer eliminates the placebo control clinical trial group
- Jan 14, 2021 – Moderna eliminates the placebo control clinical trial group
Under EUA these new products referred to as ‘vaccines’ are still undergoing data collection and thus are experimental. Placebo control safety data has now been compromised as the control group get the treatment. [1]
By March 2021 FOIA documents show 90% of placebo group had received at least one mRNA shot – i.e. “Pfizer stopped collecting useful data long before the planned end date of the clinical trial.”
Once a RCT is unblinded and the placebo control group is offered the treatment, the safety data is destroyed. “You cannot make conclusions from the data there in, after doing so.”
First COVID-19 vaccine administered in US
The first COVID-19 vaccine administered in America was to Sandra Lindsey, an ICU nurse, in New York City on December 14, 2020.
After months of vaccine development, two companies, Pfizer-BioNTech and Moderna applied to the FDA for Emergency Use Authorization (EUA). To receive approval, the companies’ data had to be reviewed by the Centers for Disease Control and Prevention (CDC) and its Advisory Committee on Immunization Practices (ACIP), which determined that health care workers and long-term care residents should be the first groups to receive the vaccine. [1, 2, 3, 4]
In a press release on December 10, 2020 the FDA assured the public that they would proceed “without sacrificing our rigorous scientific standards for safety and effectiveness.” “The FDA recognizes that transparency and dialogue are critical to building public confidence in COVID-19 vaccines.” “The FDA is considered the “gold standard” regulator of medical products. The process that the FDA uses to review is respected worldwide…”
On December 10, 2020 The FDA’s Center for Biologics Evaluation and Research’s Vaccines and Related Biological Products Advisory Committee (VRBPAC), “made up of independent scientific and public health experts from around the country”, met in open session to discuss the request for EUA of a “COVID-19 vaccine from Pfizer, Inc. in partnership with BioNTech Manufacturing GmbH” (Pfizer-BioNTech) “for the prevention of COVID-19 in individuals 16 years of age and older.” Which they approved on December 11, 2020..
ACIP met December 11& 13, 2020 “to review the Pfizer-BioNTech vaccine and recommended moving forward with its distribution to anyone over age 16. The FDA issued an EUA on Saturday 12th following the meeting and notified the CDC and Operation Warp Speed to coordinate distribution plans.”
On December 17, 2020 VRBPAC met again to discuss the EUA of the Moderna COVID-19 Vaccine “for the prevention of COVID-19 in individuals 18 years and older.” The following day on December 18, 2020 the FDA grants EUA to Moderna. [5]
Americans were assured by Operation Warp Speed that “Vaccines will help prevent the spread of COVID-19 and bring this pandemic to an end”
The CDC state that “Clinical trials provide data and information about how well a vaccine prevents an infectious disease and about how safe it is. The FDA evaluates these data, as well as manufacturing information, to assess the safety and effectiveness of vaccines. FDA then decides whether to approve a vaccine or authorize it for emergency use in the United States.”
FDA grants EUA for Moderna’s COVID-19 vaccine
On December 18, 2020 the U.S. Food and Drug Administration (FDA) announced they have “issued an emergency use authorization (EUA) for the second vaccine for the prevention of coronavirus disease 2019 (COVID-19) caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). The emergency use authorization allows the Moderna COVID-19 Vaccine to be distributed in the U.S. for use in individuals 18 years of age and older.” The FDA stated the “vaccine was 94.1% effective in preventing COVID-19 disease among these clinical trial participants with 11 cases of COVID-19 in the vaccine group and 185 in the placebo group.”
This comes the day after the December 17, 2020 VRBPAC meeting where they discussed the EUA of the Moderna COVID-19 Vaccine “for the prevention of COVID-19 in individuals 18 years and older.”
This EUA is granted 4 days after the first Pfizer-BioNTech vaccines was administered in the US and 10 days before the WHO declares the virus endemic the vaccines likely not to stop transmission!
2.79% of first dose recipients experienced a “Health Impact Event”
At a December 19, 2020 ACIP meeting, the CDC’s advisory board, Dr Thomas Clark presented slides which showed that more than 2 in 100 vaccinated were experiencing an adverse reaction. [1, 2] At this time “272,001 doses of vaccine have been administered” in the US, of which some healthcare workers have experienced serious reactions. [4, 5]
The CDC’s V-safe Active Surveillance for COVID-19 Vaccines data slide showed of 112,807 registrants with a recorded 1st dose, 3,150 of these experienced a “Health Impact Event” which means they were “unable to perform normal daily activities, unable to work, required care from doctor or health care professional”. That is 2.79% of vaccinated actively surveilled were adversely affected post injection of just the first dose. [3]
Israel begins it’s Pfizer COVID-19 vaccine rollout
On December 19, 2020, Israel begins it’s COVID-19 vaccine roll-out, the day after Hanukkah – “after paying a premium for supplies of the Pfizer/BioNTech vaccine” The first shot began with Prime Minister Netanyahu.
“If everyone cooperates, keeps the rules and goes to get vaccinated, we’ll get out of this and we could well be the first country in the world to emerge from this [pandemic]. Let’s do it together” said Netanyahu
By January 1, 2021, more than 10% of Israel’s population is said to have received their first dose of Pfizer’s vaccine. According to Pfizer executive Israel is ‘a sort of laboratory’ for Pfizer COVID vaccines. [1]
Four days after roll out, “the more contagious U.K. variant [later called Alpha] was detected in four people, and by February 10, 2021 “while the vaccine is preventing illness in older people, the variant now makes up about 80 percent of new cases.”
EMA grants first CMA for Pfizer COVID-19 vaccine
On December 21, 2020 the European Medicines Agency (EMA) granted Conditional Marketing Authorization (CMA) for the Pfizer-BioNTech COVID-19 vaccine (Comirnaty) following European Medicines Agency (EMA) “positive opinion, to BNT162b2 for active immunisation of individuals aged 16 years and older to prevent COVID-19, which is caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)” in people from 16 years of age. [1, 2]
They stating that “EMA’s human medicines committee (CHMP) has completed its rigorous evaluation of Comirnaty” concluding by consensus that sufficiently robust data on the quality, safety and efficacy of the vaccine are now available to recommend a formal conditional marketing authorisation.”
In 2022, leaked emails would reveal that there was “concern over accelerated timelines to ensure they would meet the ‘deadline’ for vaccine authorization at the expense of a robust assessment”, and there was great pressure placed on the EMA staff by the European Commissioner, Ursula von der Leyen, who had known close ties with Pfizer CEO. [1]
Israel is used as a pseudo “clinical trial” for Pfizer’s mRNA COVID-19 vaccine
On January 7, 2021 Israel’s (politically motivated) Prime Minister Benjamin Netanyahu announced his Operation Getting Back to Life deal with Pfizer Chairman and CEO Albert Bourla to exclusively use Pfizer’s COVID-19 vaccine product to vaccinate “all citizens of Israel over the age of 16 by the end of March and perhaps even earlier…” Making Israel “a global model state for the rapid vaccination of an entire country.” “We can do this because our health system is among the most advanced in the world”, being highly-digitalised. [1]
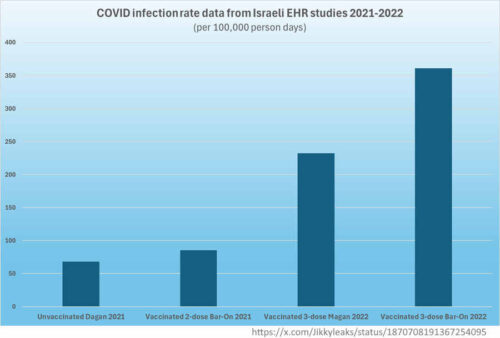
In exchange for Pfizer bringing forward vaccine deliveries, “Israel will share with Pfizer and with the entire world the statistical data that will help develop strategies for defeating the coronavirus.” – in effect making the country a mass test case to see how vaccines might halt the pandemic. [2, 7]
A new study was published in NEJM every time a booster dose was promoted for a new variant to “prove efficacy“, where each study used the less vaccinated as a control group for the “extra dose” group! [3, 4, 5, 6]
In the framework of one of the agreements “The Real World Epidemiological Evidence Collaboration Agreement” dated January 6, 2021 [8], the research outcome measures were defined by Pfizer. “None of the outcome measures that were explicitly agreed upon in advance were safety outcomes, such as overall mortality, hospitalisations from any cause or the known side effects of vaccines, whatever they may be.” [7]
“In effect, the contract between Pfizer and Israel’s Ministry of Health enabled the pharmaceutical giant to conduct a real-world experimental study of its Covid-19 mRNA vaccine efficacy using the Israeli population as test subjects”, and use it to promote alleged “effectiveness”. [7]
Pfizer had “the power under the agreement to omit any reference to its contribution to the research, so its involvement in setting research goals, methods or even in writing the research results is not mentioned at all. Thus, a study may be portrayed as independent of Pfizer, although it is not necessarily so.” [7]
TGA grants first COVID-19 vaccine Provisional Registration – Pfizer-BioNTech
On January 25, 2021 Australia’s TGA grants Provisional Registration for Pfizer Australia Pty Ltd‘s mRNA COVID-19 Vaccine for 16 years and older, the first vaccine for COVID-19 to receive “provisional approval” in Australia and the first new gene technology vaccine ever used. [1, 2, 3]
COMIRNATY (BNT162b2 [mRNA]) COVID-19 Vaccine has provisional approval for the indication below:
- Active immunisation to prevent coronavirus disease 2019 (COVID-19) caused by SARS-CoV-2
- The use of this vaccine should be in accordance with official recommendations.
- The decision has been made on the basis of short term efficacy and safety data. [clinical trial]
- Continued approval depends on the evidence of longer term efficacy and safety from ongoing clinical trials and post-market assessment.
This product, like all provisionally registered products, falls under the TGA black triangle safety monitoring scheme.
Moderna begins vaccine booster trials as evidence of “waning immunity” emerges
In a January 25, 2021 press release Moderna announce the “waning immunity” potential of their COVID-19 vaccine against mutating SARS-CoV-2 variants, in particular to the South African variant B.1.351 which is later called Beta. [1]
“Out of an abundance of caution and leveraging the flexibility of our mRNA platform, we are advancing an emerging variant booster candidate against the variant first identified in the Republic of South Africa into the clinic to determine if it will be more effective to boost titers against this and potentially future variants.” said Stéphane Bancel, Chief Executive Officer of Moderna, confirming talk from earlier in the month about boosters.
Moderna announced they will:
- First, “test an additional booster dos0 “of its current COVID-19 Vaccine (mRNA-1273) to study the ability to further increase neutralizing titers against emerging strains beyond the existing primary vaccination series.
- Second, they would begin “an emerging variant booster candidate (mRNA-1273.351) against the B.1.351 variant…into preclinical studies and a Phase 1 study in the U.S. to evaluate the immunological benefit of boosting with strain-specific spike proteins.
- Moderna expects either one will “further boost neutralizing titers in combination with all of the leading vaccine candidates.”
CDC begins defining a “vaccine breakthrough case” as “vaccine failure” becomes obvious
On January 27, 2021 the CDC (in an email obtained under Freedom of Information) defined a COVID-19 “vaccine breakthrough case” as
“a patient who has SARS-CoV-2 RNA or antigen detected on a respiratory specimen collected [greater than or equal to] 7 days after completing the primary series of an FDA-authorized SARS-CoV-2 vaccine” [1, 2]
Though in retrospect, we learn CDC’s Dr Fisher as early as December 21, 2020, only 7 days after rollout begins, he was directed by a superior “to start working on a protocol to evaluate COVID vaccine failures or breakthrough cases.” Also on January 30, 2021, CDC Director, Dr. Rochelle Walensky, began planting concern about virus variants being a “growing threat” of escaping the protection of vaccines.
On January 27, 2021 at the CDC a 1-page internal document about “vaccine failure” was being distributed by CDC medical officer Dr. Thomas Clark the Epoch Times reveals, but under FOIA it is fully redacted.
On February 2, 2021 in an email, the CDC’s Vaccine Breakthrough Case Investigation Team, led by Dr Fisher and part of the COVID-19 Vaccine Taskforce, alters the definition of a breakthough case to be least 14 days post the completion of a primary dose series of injections. This instantly eliminates cases and provides an inflated and misleading view of vaccine effectiveness. [3]
“A US. resident who has SARS-CoV-2 RNA or antigen detected on a respiratory specimen collected [greater than or equal to] 14 days after completing the primary series of an FDA-authorized COVID-19 Vaccine.”
On February 4, 2021 the CDC communicated with US States on how to count vaccine breakthrough cases and which to exclude!
It wasn’t until April 15, 2021 that the CDC began reporting vaccine Breakthrough Cases, which included some who were hospitalised and died. [4, 5]
On May 1, 2021 the ” CDC transitioned from monitoring all reported vaccine breakthrough cases to focus on identifying and investigating only hospitalized or fatal cases due to any cause”. [6, 7]
In an email to the Epoch Times it was claimed the “CDC made the change to the definition of a breakthrough infection time period due to the most current data that showed that the 14-day period was required for an effective antibody response to the vaccines.” At this point the CDC are assuming antibody production is equivalent to “preventing infection”, a theory. They claimed to have wanted to “eliminate cases where exposure [to the virus] happened before the vaccination response [antibody production] would be effective.”
As Dr Harvey Risch points out to Epoch Times, “If the vaccines don’t work for the first 7 or 14 days or increase risk of getting COVID-19 during that period, that is part of what happens when they [the mass vaccination program] are deployed in a population.”
Dr Jay Bhattacharya told The Epoch Times in an email, the CDC should have warned “recently vaccinated vulnerable older people that they were at higher risk for being infected during that period.”
Japan approves first COVID-19 vaccine – Pfizer-BioNTech
Japan’s Ministry of Health, Labor and Welfare gave the first “fast-track approval ” to Pfizer-BioNTech COVID-19 vaccine on Sunday February 14, 2021 in ages 16 years and older. Stated as “95 percent effective at preventing symptoms of COVID-19”. [1, 2]
On Wednesday February 17, 2021 it began rollout first with 40,000 healthcare workers who will receive two shots to be administered three weeks apart. “Of the initial group of health workers, 20,000 will participate in a study to track side effects potentially caused by the vaccine.”
“A further 3.7 million front-line health workers are to begin receiving the vaccine in March, followed by 36 million people aged 65 or older from April” 2021. ” People with pre-existing conditions such as diabetes or heart disease and those working at elderly care facilities will come next, and then finally the general population.”
- COVID-19 vaccinations began 6 months out from the start of the 2021 Tokyo Olympics and Paralympics
- In 2020 Japan had no excess death above expected.
- By April 21, 2021 – Japan PM discussed receiving 50 M additional Pfizer doses, on top of existing agreements for 144 M doses – total 194 M doses, enough for 97 M people.
- By May 2023 Japan’s CV19 vaccine dose orders totalled: Pfizer 194M, Moderna 100M, Oxford/AstraZeneca 120M, Novavax 150M, of that 564M total, 10.24M doses were donated to other countries. This includes booster shots. With Japan’s population in 2021 as 125.6M, the remaing doses equate to 4.4 shots for every single person.
TGA grants Provisional Registration for AstraZeneca COVID-19 Vaccine
On February 16, 2021 the Australian TGA grants Provisional Registration for the AstraZeneca COVID-19 Vaccine, the second gene-based vaccine approved for use in Australia.
COVID-19 Vaccine AstraZeneca has provisional approval for the indication:
- Active immunisation of individuals ≥ 18 years old for the prevention of coronavirus disease 2019(COVID-19) caused by SARS-CoV-2.
- The use of this vaccine should be in accordance with official recommendations.
- The decision has been made on the basis of short term efficacy and safety data. [Clinical Trial]
- Continued approval is dependent upon the evidence of longer-term efficacy and safety from ongoing clinical trials and post-market assessment.
“As a provisionally registered product, this medicine will remain in the Black Triangle Scheme for the duration of its provisional registration.”
The TGA made a safety assessment based on clinical trials that used a meningococcal vaccine (MenACWY) as a control, and not an inert, saline placebo.
“The world is engaged in the largest clinical trial, the largest global vaccination trial ever”
On February 21, 2021, in an ABC interview with David Speers, the Australian Health Minister, Greg Hunt noted, [1, 2]
“The world is engaged in the largest clinical trial, the largest global vaccination trial ever, and we will have enormous amounts of data.” said The Hon Greg Hunt MP [3]
Hunt also said:
“One of the things that is absolutely fundamental to confidence is the belief in safety. And the essence of safety is a full and thorough assessment. We know that from all of our research that in order to increase confidence, you need a strong belief in safety. “
This HERE is the Pfizer document FOIA assessment. Did Australia’s TGA do a “full and thorough assessment”?
How do you instil “belief”? explore controlling the narrative via censorship – HERE
COVID-19 vaccine roll-out begins in Australia
On February 22, 2021 together Australia’s Prime Minister, the Chief Medical Officer, and the Chief Nursing and Midwifery Officer, officially launched Australia’s COVID-19 vaccination program for groups at higher risk of death due to COVID-19.
Johnson & Johnson Vaccine receives US EUA, Australia rejects it’s use.
On February 27, 2021, following VRBPAC‘s green light, the US FDA granted the Johnson & Johnson (J&J) COVID-19 Vaccine Emergency Use Authorisation (EUA) for 18 years and older. To following day, Feb 28, the CDC’s ACIP committee voted unanimously to recommend the vaccine in the US population. [1]
The Janssen/J&J COVID-19 Vaccine is an adenovirus vector-based vaccine which carries the DNA code of a stabilized SARS-CoV-2 spike into the human cell. It is the first COVID-19 vaccine approved as a single dose.
In Australia, in early April it was announced that “Johnson & Johnson’s one-dose vaccine will not be part of Australia’s vaccine rollout…Health Minister Greg Hunt said that “similarities” to AstraZeneca’s vaccine “were the reason the federal government had decided against pursuing the option any further.” [1]
By April 2021 enough recipients of AstraZeneca’s vaccine was experiencing blood-clotting issues in the under 50 age group, to steer authorities to recommend Pfizer’s mRNA vaccine instead.
By May 5, 2022, the U.S. FDA limited the authorised use as the vaccine a is may cause “thrombosis with thrombocytopenia syndrome (TTS) which may be life-threatening.”
TGA Access Consortium decide changing the “vaccine” mRNA code does not make it a new product!
On March 5, 2021 Australian TGA’s Access Consortium are considering changing the COVID-19 vaccine’s mRNA code during the pandemic, in a provisionally registered vaccine, and compare is to seasonal influenza vaccine changes, even though they are completely different technologies.
“On public health and scientific considerations, Regulatory Authorities do not consider an updated coronavirus vaccine to be an entirely novel product with the resulting requirement for lengthy full-blown clinical studies.”….”a regulatory approach like for seasonal updates for influenza vaccines can be taken”
“Since an updated vaccine variant will build on a previously authorised parent version with established quality, safety and efficacy; from a public health perspective, it may be justifiable to roll out the new vaccine candidate already in parallel with the previous version in absence of clinical immunogenicity and safety data while these studies are ongoing.”
It appears that since a “booster” has been granted TGA provisional approval, changing the mRNA code may not require much regulatory oversight!!! Yet a different protein could have profound cytotoxic implications.
It appears the regulatory agency is authorising the “vaccine platform” and not the “active ingredient” which mRNA code will force the body to manufacture. The body makes the “active ingredient”, that is unique for each vaccine, but apparently not according to the regulators.
Pfizer add booster assessment to ClinicalTrials.gov
Just over 3 months into the vaccine roll out in the U.S., on March 20, 2021, Pfizer updated their original clinical trial mRNA vaccine protocol to include a new assessment criteria for a third dose (booster). “In order to describe the boostability of BNT162… against emerging SARS-CoV-2 VOCs, an additional dose of BNT162b2 will be given to Phase 1 participants approximately 6 to 12 months after their second dose…” [1, 2]
The mRNA vaccines were only assessed for reducing symptoms to COVID-19, and many vaccinated individuals are getting COVID-19, including severe symptoms – meaning the vaccines are “leaky“. The COVID-19 vaccines do not stop transmission or infection, and were never assessed for this, they were assessed on symptoms.
On April 1st, 2021 Pfizer then added “and potentially a fourth dose of prototype BNT162b2VOC (BNT162b2s01, based upon the South African variant and hereafter referred to as BNT162b2SA).”
Are the mRNA synthetic codes in these “vaccines” being altered? Is that what the “b1”, “b2” “b2SA” etc mean? This appears to be setting the stage for boosters every 6 months with a “new” synthetic mRNA code.
COVID-19 vaccines create 9 new billionaires
On May 20, 2021 Oxfam released a report titled, “COVID vaccines create 9 new billionaires with combined wealth greater than cost of vaccinating world’s poorest countries”. [3, 4]
Topping the list are the CEOs of BioNTech and then Moderna. The top 2 to 6 are linked to Moderna. Neither company had brought a product to market prior to their brand new technology COVID-19 mRNA vaccine. [1, 2]
The last 3 billionaires are co-founders of CanSino Biologics, manufactures of China’s Ad5-nCoV-S recombinant (Ad5-nCoV) vaccine against COVID-19. [3]
CDC acknowledge increasing numbers of myocarditis VAERS reports
The May 27, 2021 CDC’s COVID-19 vaccine Adverse Events update first acknowledged myocarditis, stating in “April and May of 2021, there have been increased reports to the Vaccine Adverse Event Reporting System (VAERS) of cases of inflammation of the heart—called myocarditis and pericarditis—happening after COVID-19 vaccination in the United States. These cases have been reported after vaccination with the Pfizer-BioNTech and Moderna COVID-19 vaccines and have mostly been reported in adolescents and young adults.” [1]
FOI request shows vaccine LNP does NOT stay at the injection site
On May 28, 2021 Dr Byram Bridle, a vaccine expert and part of the Canadian COVID Care Alliance released a warning statement on the COVID-19 vaccine safety, in particularly referencing a Pfizer biodistribution study from Japanese regulatory authorities [translated] which shows the PEG-coated synthetic lipid nano-particles (LNP) travel through the blood and accumulate in organs, especially in the ovaries. Demonstrating that the “vaccine” can distribute the mRNA code to any body system to start producing the spike protein at that location. [1, 2, 3, 4]
Further to this, Dr Robert Malone, the founder of the mRNA technology, when interviewed by the German Corona Investigation, voiced his concerns that Luciferase code was used to “demonstrate” that the vaccine stays at the injection site. He added that the “regulatory authorities are not sufficiently technically expert to comprehend” that they were being deceived by the data presented to them by the vaccine manufacturer. For LNP to accumulate in all body organs, means it does not stay at the injection site as was told. [5]
With the biologically active spike protein (manufactured by the body of the vaccinated) circulating in the blood (at lease potentially) what measures are the red cross taking for tracking blood donations? In Australia, wait 7 days before donating because of side effects.
Australia’s TGA uses data from FDA and equivalent international regulators when assessing products to register for Australians.
FDA Approves First Oral Blood Thinning Medication for Children
On June 21, 2021 the FDA approved the first oral blood thinning medication, Pradaxa (dabigatran etexilate) oral pellets, to treat children 3 months to less than 12 years old who have venous thromboembolism (a condition where blood clots form in the veins) directly after they have been treated with a blood thinner given by injection for at least five days. The only other approved blood thinning medication for children is given by injection.
In November 2021 the dose-reduced vaccine rollout in children 5-11 years began. [1]
Is this getting ahead of the expectation that children will develop blood clots to the COVID-19 vaccines, and they can be treated at home – the press say no because it is not approved in children and “There’s no evidence connecting the Pfizer vaccine to blood clots”!
FDA adds myocarditis warning for mRNA vaccines
On June 25, 2021 the US FDA announced the addition of a warning “to the patient and provider fact sheets for the Moderna and Pfizer-BioNTech COVID-19 vaccines regarding the suggested increased risks of myocarditis (inflammation of the heart muscle) and pericarditis (inflammation of the tissue surrounding the heart) following vaccination.” [1]
TGA grants Provisional Registration to J&J COVID-19 vaccine
Janssen Pharma who is wholly-owned by Johnson & Johnson (J&J), On June 25, 2021 were granted provisional registration by the TGA for their “COVID-19 Vaccine Janssen” for use in 18 years and over. This is the third “provisional use” vaccine in Australia, and the first one-dose COVID-19 vaccine, which was first submitted for determination on November 16, 2020. [4]
The active ingredient is “adenovirus serotype 26 encoding the SARS-CoV-2 spike glycoprotein (Ad26.COV2.S)” as stated in the public assessment report, together with a list of it’s excipients. [1, 2, 3]
The approval decision was made on preliminary short term efficacy and safety data, and is thus under ongoing clinical and post-market assessment under black triangle scheme.
FDA warns of increased risk of heart inflammation post-jab
On June 25, 2021 the FDA update the patient and provider Fact Sheets for Moderna & Pfizer-BioNTech‘s mRNA COVID-19 vaccine to warn and reflect “the suggested increased risks of myocarditis (inflammation of the heart muscle) and pericarditis (inflammation of the tissue surrounding the heart) following vaccination”…”particularly following the second dose and with onset of symptoms within a few days after vaccination.” [1, 2, 8]
Two days prior, on June 23, 2021 ACIP met to discuss the rising incidence of myocarditis (heart inflammation) but despite alarming data, [5] they didn’t advise to halt the vaccine in this age group. [4]
Since March 2021 there has been documented increasing incidences of fit and healthy athletes suddenly collapsing and dying, but also in the general population. [6, 7]
Dr McCullough, a cardiologist, says there’s no such thing as “mild” myocarditis from the vaccines. Once heart cells are damaged, that’s permanent. An elevated troponin level from natural infection is transient and should not be confused with vaccine induced myocarditis. [3]


