This page plots the timeline of events that led up to the TGA’s reform allowing for a genetically modified synthetic gene sequence encapsulated by a synthetic lipid to be considered a “vaccine” and plugged into a relatively newly formed regulatory pathway. A regulator, who’s role is to protect it’s people, but is funded by the sponsor, allowed a gene technology product, referred to as COVID-19 mRNA vaccines, to enter the newly legislated provisional registration pathway, which in record time, worked its way to full registration which now by default is somehow considered a vaccine platform. How on earth did we get here, that is what I’m attempting to plot.
Since 2022 the TGA, just like the WHO and FDA, have updated their website and deleted many pages, which makes it difficult to locate information, so be aware many links below are web archived pages. You’d think public health were trying to make it difficult for the public to find vaccine information!
There is no legal definition for “vaccine”
- IMPORTANT: No where in Therapeutic Goods Act or Regulations is there a definition for what constitutes a “vaccine” product, as such there is no legal definition. Yet the regulations call for an Advisory Committee on Vaccines (ACV)!
- According to the regulations “vaccines” fall under the category of “Biological Medicine“, but not the blood/tissue product type of Biological.
- The regulations have a definition for “gene therapy” products, yet mRNA “vaccines” some how escaped that category, yet fit the definition perfectly.
- It appears if a sponsor refers to their product as a “vaccine” then it is considered a vaccine by the regulator!
- It appears if the mRNA code encodes for an antigen protein, that is all that is needed for it to be considered a “vaccine”, if it encodes a human derived protein it will likely be considered a “drug”. It’s all in the code (as far as I can work out! – Totally shocking!)
To fully understand the TGA we need to also look at the history of the Department of Health in Australia, of which the TGA falls under.

Australian Register for Therapeutic Goods (ARTG) – HERE, ARCHIVE
Read more for youreself:
- History of TGA – PDF
- History of Clinical Trials regulation Australia – HERE
Brief History of TGA
1911- Commonwealth Vaccine Depot (CVD) was established
1916 – CVD became Commonwealth Serum Laboratories (CSL) during war time to provide a source of anti-toxins and other biological agents, it took over manufacture of smallpox vaccine. [1, 5]
- CSL is privatised in 1991, sold in 1994 to private investors for $299 million [2] now worth $145 billion [3]
- 1956 CSL mass produced polio vaccine [6]
1921 – Department of Health was established [4]
1937 – Therapeutic Substances Act 1937 giving Minister of Health drug control powers
1953 – Therapeutic Substances Act 1953 administered by Dept of Health
1953 – National Biological Standards Laboratory (NBSL) established to monitor imported products
1989 – Therapeutic Goods Act 1989 – HERE
1989 – Therapeutics Goods Administration (TGA) was created as a Division of the Department of Health equivalent
1990 – Therapeutic Goods Regulations 1990 – HERE
2001 – Office of Gene Technology Regulator (OGTR) established out of Act and Regulation – MORE
TGA: Definition of Gene Therapy
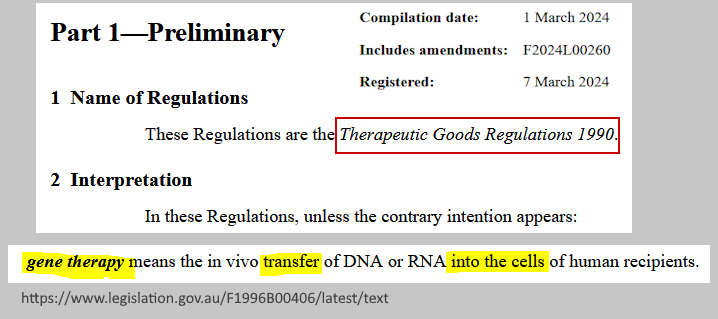
“gene therapy means the in vivo transfer of DNA or RNA into the cells of human recipients”
(nothing is stated about integrations into DNA) – source, PDF
Documents and links below help provide a thread of understanding of the local and global regulatory web and “justification” for consolidation, which they call “harmonisation”. It all sounds good to streamline the regulation of the same product around the world, until you realise the decisions are distantly related to “health” but closely controlled or influenced by those who profit.
Listed in reverse chronological order
This timeline is a work in progress, content will be added as time permits
2024
January 17, 2024 – The European lobby Kangaroo Group, hosted a lunch in the European Parliament with Members with BioNTech and Moderna as key speakers, to discuss the forthcoming revision of “European Commission’s proposal for a reform of the EU General Pharmaceutical Legislation (GPL)”. EU- ARCHIVE, KG – ARCHIVE, PDF, CREDIT
- Their objective to effecttively change the definition of gene therapy, underwhich their COVID-19 mRNA vaccines are classified, and to create a “definition of Platform Technologies, as well as a clear demarcation between Gene Therapies Medicinal Products that alter human genomes, and those that do not (e.g. mRNA).”
- [If it is happening in the EU, it’s likely happening with all regulators]
2023
February 24, 2023 TGA – Priority Review pathway for biologicals: feasibility, potential eligibility criteria and determination process – submissions – READ, Priority review – WEB, Priority Review of Biologicals – READ, Application Process – READ
- [It’s unclear when the Priority Review legislation was updated as the TGA no longer add dates to their web pages, like they’re trying to hide something, at the very least make it harder to track their changes.]
- Consultation opened March 28, 2022 – REA
2022
August 1, 2022 – Journal of Law and Medicine: A new priority pathway for biologicals in Australia: contextualising and evaluating the proposed reforms by Rudge et al – READ, READ
- Examines recent reforms to the regulatory framework for biologicals contained in the Therapeutic Goods Act 1989 (Cth) in the context of the “New Frontier” of reform envisioned in a report completed by the Commonwealth Government in 2021….identifies several potential shortcomings of the proposed reforms and reports on the current lack of data on the processes of expedited approvals in Australia more generally.
July 4, 2022 – TGA Advanced Therapies: Report on ‘Cell, Gene and Tissue Regulatory Framework in Australia – A stakeholder engagement report on Advanced Therapies prepared for the Therapeutic Goods Administration – READ, Report – PDF, ARCHIVE
- In November 2021 the Therapeutic Goods Administration (TGA) commissioned Medical Technologies and Pharmaceuticals Growth Centre (MTP Connect) to conduct a stakeholder review of the regulatory framework for gene, cell and tissue therapies in Australia.
May 23, 2022 – ARCS Annual Conference 2022: Regulation of cell and gene therapies in Australia – Presentation – PDF
- Biological Medicines (prescription medicines) – vaccines (that do not contain viable human cells)
- February to April 2022 – TGA initiated a public consultation – support the introduction of a new Priority Review pathway for biologicals
- Consideration for priority – the biological is to be used for treatment, prevention [AKA VACCINE!] or diagnosis of a life threatening disease or seriously debilitating condition (as described by the TGA)
January 10, 2022 – TGA – New catagory appears called Advanced Therapies, under which gene therapies suddenly appears! – ARCHIVE, READ, Quitely added to the website with NO press release or statement – ARCHIVE, unable to find regulatory change around this time – READ
2021
June 4, 2021 – June 4, 2021 – Legislation: Therapeutic Goods Amendment (Therapeutic Goods Advertising Code) Instrument 2021 – ARCHIVE – It is now “legal” or permissible to advertise COVID-19 vaccines “safe” in Australia – TIMELINE
- Australia’s Therapeutic Goods Advertising Code is amemded to be able to claim COVID-19 vaccines as “safe” – (Dr Philip Altman) – REF [As I am not a legal person it is unclear which instrument brought this advertising permission into effect, it appears to be June 4, 2021 set off the snowball]

- June 4, 2021 – Dept Health: Therapeutic Goods (Restricted Representations – COVID-19 Vaccines) Permission 2021 – Skerritt’s letter – (now Repealed) READ, no early ARCHIVE
- “These arrangements have been enabled by the Therapeutic Goods (Restricted Representations—COVID-19 Vaccines) Permission 2021 made on 4 June 2021. . An amendment to the Therapeutic Goods Advertising Code (No.2) 2018 – external site) has also been made to remove the requirement for self-developed advertising to comply with the Code, as the critical requirements have been covered in the above Permission.”
- relevant COVID-19 vaccines means registered goods – where “registered” includes experimental provisional goods!
- June 7, 2021 – Dept Health: New regulatory arrangements support businesses and health professionals to communicate and incentivise COVID-19 vaccination – READ, ARCHIVE,
- September 23, 2021 – Legislation change: Therapeutic Goods (Restricted Representations – COVID-19 Vaccines) Permission (No. 4) 2021 – READ , ARCHIVE
- Authority: This instrument is made under section 42DK of the Therapeutic Goods Act 1989
- “In recognition of the importance of responsible communications regarding the COVID-19 vaccination program, the TGA has issued a subsequent legal permission (the 2021 permission) that allows advertisers such as health professionals, participating vaccination sites, corporate entities, media outlets and others to develop their own materials to communicate publicly about COVID-19 vaccines subject to the conditions below.” – REF

Legislation: Therapeutic Goods (Therapeutic Goods Advertising Code) Instrument 2021 –READ
- November 8, 2021 – TGA: Communicating about COVID-19 vaccines – ARCHIVE
- The following guidance explains how parties can lawfully provide communications about COVID-19 vaccines to support the Government’s COVID-19 vaccine roll-out.
- November 29, 2021 – Legislation: Therapeutic Goods (Therapeutic Goods Advertising Code) Instrument 2021 – ARCHIVE
- July 1, 2022 – The Therapeutic Goods Advertising Code (the Code) is the cornerstone of the therapeutic goods advertising regulatory framework – ARCHIVE
- “From 1 July 2022, advertisers must ensure their advertisements comply with the Therapeutic Goods (Therapeutic Goods Advertising Code) Instrument 2021 (2021 Advertising Code).”
April 21, 2021 – Monash Uni: Monash supports Australia’s first mRNA vaccine facility – ARCHIVE
- The Victorian government has announced it will put $50 million towards the first domestic production of mRNA COVID-19 vaccines, such as Pfizer and Moderna…
- Experts from Monash University’s Biomedicine Discovery Institute will also work on the project, including mRNA specialists from the fields of biotechnology, infectious disease, immunity and cancer.
- The proven scalability and comparative low-cost vaccine manufacturing potential of mRNA vaccines and nanomedicines secures them as the critical technology platform for vaccines and medicines of the future.
- Increasingly, mRNA nanomedicine can also be used in the treatment of cancer, rare diseases, cellular engineering and protein-replacement therapy, so this investment will help accelerate mRNA projects and develop new treatments to save lives.
2019
November 21, 2019 : Good Manufacturing Practice (GMP) for new and emerging technologies: Advanced Therapy Medicinal Products (ATMPs) – Presentation – ARCHIVE, PDF
September 27, 2019 – TGA business plan 2019-20 – ARCHIVE, PDF
- “The Business Plan sets out our product regulation, regulatory reform, international engagement and regulatory compliance agenda for 2019-20 and the steps we will take to achieve our vision.”
Better health and wellbeing for all Australians through regulatory excellence
TGA Vision statement
- Our priorities are derived from:…TGA International Engagement Strategy 2016-2020 – READ (which began in 2013)
- TGA “proactively responding to innovations in therapeutic goods, which may require updates to regulation all identifiable opportunities for international work sharing and harmonisation.”
- “The TGA annual budget is approximately $165 million and we operate predominantly on a cost recovery basis. The regulatory costs are recovered through fees and charges levied on sponsors and manufacturers of therapeutic goods…” – REF

- The Australia-Canada-Singapore-Switzerland (ACSS) Consortium‘s work sharing pilot on New Chemical Entities is a unique global collaboration between regulatory authorities and the pharmaceutical industry.
August 12, 2019 – Dept Health: Australian Health Management Plan for Pandemic Influenza (AHMPPI) – READ, ARCHIVE, PDF
- AHMPPI, the national government health sector pandemic influenza plan, outlines the agreed arrangements between the Australian Government and State and Territory Governments for the management of an influenza pandemic. This agreement was ignored a few months later when another respriatory virus pandemic was declared in 2020!
- The previous version of this plan was 2008, which conincidentally a year later the 2009 H1N1 pandemic was declared. The Australian govt did successfully utilised the plan that year.
June 2019 – TGA Annual performance statistics report: July 2018 to June 2019 – “provides detailed statistical information on our performance to our stakeholders.” – ARCHIVE, (2016-2019) – ARCHIVE
- “At 30 June 2019 there were 88,788 therapeutic goods on the ARTG, including 31,987 new products added during the reporting period. All therapeutic goods registered on the ARTG can be lawfully manufactured and supplied in Australia and include prescription medicines, over-the-counter medicines, complementary medicines, biologicals, and medical devices.” [curiously no mention of vaccines, which are a separate category called biological medicines, separate from bioligicals]
- Thus 36% of all registered products listed on ARTG were added in one financial year!
February 8, 2019 – Prescription medicine major variation: discretionary power to reduce fees in exceptional circumstances – ARCHIVE
- The sponsors fee reduction is only intended for safety related changes in certain circumstances.
2018
December 2018 – TGA release a promotional video below, … they’re “looking out for you.” – WATCH, ARCHIVE
August 2, 2018 -TGA: Priority review pathway: prescription medicines- Information on the priority review pathway for the registration of novel prescription medicines – READ
June 29, 2018 – TGA Advertising Code opens up definition for a “serious form of a disease” “to be anything deemed “medically accepted” as a serious disease or a diagnostic test available” – TIMELINE
March 29, 2018 – Provisional Registration added to TGA legislation – TIMELINE
- “On March 29, 2018 the Australian government added a Provisional Registration (PR) amendment to the Therapeutic Goods Act (1989), of which the TGA Secretary now has overriding power to send a product application through the registration process with only preliminary clinical data.”
March 23, 2018 – TGA: Completing a designation or determination extension application form in TGA Business Services – A step-by-step guide for agents and sponsors who wish to apply for an extension of a previously approved provisional determination – ARCHIVE, SOURCE
March 21, 2018 – TGA: Australian Regulatory Guidelines for Prescription Medicines (ARGPM) -UPDATE: Implementation of a Provisional approval pathway for the time limited registration of eligible prescription medicines now avaliable – ARCHIVE
March 20, 2018 – TGA announce time-limited provisional registration pathway is now available – ARCHIVE, TIMELINE
- ” The provisional approval pathway allows sponsors to apply for time-limited provisional registration on the Australian Register of Therapeutic Goods (ARTG)
- “As part of the Government’s response to the Review of Medicines and Medical Devices Regulation (MMDR review), we are implementing a pathway for the provisional registration of prescription medicines. Approval through the provisional pathway will be on the basis of preliminary clinical data where there is the potential for a substantial benefit to Australian patients.
- The provisional approval pathway allows sponsors to apply for time-limited provisional registration on the Australian Register of Therapeutic Goods (ARTG). It provides access to certain promising new medicines where we assess that the benefit of early availability of the medicine outweighs the risk inherent in the fact that additional data are still required.”
March 6, 2018 – TGA legislations change sets up for government to advertise “vaccines” on TV in the interest of public health –TIMELINE
January 2, 2018 – TGA announce Comparable overseas regulators (CORs) for prescription medicines – Criteria, COR report-based process and work-sharing – ARCHIVE, SOURCE
- “In response to the Medicines and Medical Devices Review (MMDR) we are reforming the way we collaborate with comparable overseas regulators” – and “implementing a formal process for work-sharing with CORs on prescription medicines applications.”
- Under this process, multiple regulators would be able to work simultaneously on different parts of a dossier submitted for evaluation in each jurisdiction. A joint evaluation report would then be provided to each agency to allow independent decision-making.”
- They began with re-assessing generic medicines – Nov 2017 ACSS Consortium and the Generic Medicines Work-Sharing Trial presentation – ARCHIVE
2017
June 26, 2017 – TGA: Priority review pathway for the registration of novel prescription medicines for Australian patients, providing “faster assessment of vital and life-saving prescription medicines” – ARCHIVE, ARCHIVE
- The very pathway all COVID-19 vaccine entered to gain regulatory handshake
- Sponsors lodge their application first for TGA Secretary to determine whether the medicine/vaccine is eligible for registration via the Priority review pathway, once approved it enters the provisional pathway.
March 2017 – TGA: Expert Panel Review of Medicines and Medical Devices Regulation 2016 – Streamlining Statutory TGA advisory committees – ARCHIVE
- Efficiencies will be achieved through reducing the number of statutory advisory committees that provide independent expert advice to the TGA. – i.e. Advisory Committee for Vaccines (ACV) funded by the sponsor “cost arrangement” – TIMELINE
January 2, 2017
2016
September 15, 2016 – TGA: Australian Government Response to the Review of Medicines and Medical Devices Regulation – The case for reform presented by the Expert Panel – ARCHIVE, Reforms proposed – ARCHIVE
- “The Expert Panel identified several significant trends in the regulation of medicines and medical devices internationally. In particular,…allowing earlier access to medicines … through the development of provisional approval pathways” There are “benefits of harmonising international regulatory frameworks”.
- “TGA has a strong reputation as a regulator both domestically and internationally, and benchmarks well against comparable overseas regulators. However, …there are opportunities for reform and improvement in the regulation of therapeutic goods.”
- “…allowing for greater flexibility in approval pathways …(including greater use of overseas assessment reports and provisional approvals in certain circumstances) would expedite access to market without compromising the safety, quality and efficacy or performance of medicines and medical devices.” [Bold claim!]
- “The Panel also identified areas of regulation where a more risk-based approach could be adopted to more appropriately align regulation with the risk posed by regulated products” [Do you sense they are suggesting vaccines here, which are believed to be, by default of their name, safe and effective” ?]
May 2016 – The Australian Government Response to the Review of Medicines and Medical Devices Regulation – PDF, ARCHIVE
- Recommendation 10 – Provisional: “Subject to the provision of clear advice to consumers and health practitioners that the medicine has been granted provisional approval and the implications of that for the consumer/health practitioner”
- [Was it made clear th the pubic and health practitioners that the COVID-19 vaccines in Australia were “Provisional” when released in 2021?]
2015
November 2015 – Australian Goverment establishes Medical Technologies and Pharmaceuticals Growth Centre (MTP Connect) – as part of the $248 million Industry Growth Centres Initiative, an alleged independent, not-for-profit organisation – ARCHIVE , Gov- ARCHIVE, Factsheet – PDF
- MTP Connect Chair, Dr Bronwyn Evans – was from 2009-2012, the Chair of the Medical Technology Association of Australia (MTAA), a national peak industry body representing companies in the medical technology industry…for a healthier Australia..and “also play a vital role in providing healthcare professionals with essential education and training” – of non-pharmaceutical products. REF
July 31, 2015 – Review of Medicines and Medical Devices Regulation: Recommendations to the Minister for Health on the Regulatory Frameworks for Medicines, Medical Devices, Complementary Medicines and Advertising of Therapeutic Goods – Stage TWO Review report – PDF, PDF, ARCHIVE
- “The rapid pace of innovation and change in the health sector confers both benefits and risks. The Government’s deregulation agenda is based on the core principle that well-designed regulation has a role in achieving the greatest net benefit for the Australian community….positioning Australia to effectively respond to emerging opportunities for, and manage challenges to the health and safety of the public”
March 31, 2015 – Expert Panel Stage ONE Review report and discussion papers – PDF, ARCHIVE
2015 – TGA Acronyms & Glossary – Definitions – ARCHIVE, READ
- Active Ingredient
- Anticeptic
- Bacterial endotoxin limit
- Bioburden
- Biological
- Biological Medicine (vaccine)
- Cochrane review
- Common Technical Document (CTD) format – An internationally agreed set of specifications for a submission dossier….
- Disease
- Drug – See medicine (Section 3(1) of TG Act
- Genetically Modified Organism (GMO)
- Homoeopathic preparation
- Informed consent
- “In relation to treatment or proposed treatment, means consent freely given by a person on the basis of information concerning the potential risks and benefits of the treatment that was sufficient information to allow the person to make an informed decision whether to consent to the treatment”
- Medicine
- (no definition for “Gene Therapy” but can be found in the TG Regulations)
- (no definition for vaccine, can’t be found anywhere!)
- Medical Device – READ
- New chemical entity (NCE) – could be anything!
- Placebo
- “An inactive substance or treatment that supposedly has no treatment value. It is given to participants in clinical trials as a control against which to compare the effects of the test substance. In practice, placebos may also have positive or negative effects on trial participants.”
- Control
- “In clinical trials comparing two or more interventions, a control is a person in the comparison group that does not receive the medicine or treatment under evaluation. Instead that person receives a placebo, no intervention, usual care or another form of care. In case-control studies, a control is a person in the comparison group without the disease or outcome of interest.”
- Therapeutic goods
2015 – Legislation Australian Immunisation Register Act 2015 – NEED MORE INFO
- recognised vaccination provider within the meaning of the Australian Immunisation Register Act 2015 – REF
2014
October 28, 2014 – Dept. Health: Expert Review of Medicines and Medical Devices Regulation announced – ARCHIVE, Web page ARCHVIES
- Media Release: Health Minister Peter Dutton and Assistant Minister for Health Fiona Nash announced they had appointed an Expert Panel Review of Medicines and Medical Device Regulation – PDF
- “We need a modern regulatory framework to ensure Australians can access the latest treatments in a timely manner,” Dutton
- The scope of the Review is set out in the Terms of Reference, review report due March 31, 2015 – PDF
- The Review panel experts are Emeritus Professor Lloyd Sansom AO (chair), Professor John Horvath AO, Mr Will Delaat AM
June 5, 2014 – The Health Ministerial Advisory Council – ARCHIVE
- Health Ministerial Advisory Council (Health MAC) has been established to provide advice to the Health Ministers about opportunities to reduce regulatory and red tape burden within the Health portfolio. Health MAC falls within the Deregulation Branch, Best Practice Regulation and Deregulation Division of the Department of Health.
- Chair: Hon Peter Dutton MP, Minister for Health and Minister for Sport
- Deputy Chair: Senator the Hon Fiona Nash, Assistant Minister for Health
April 22, 2014 – Dept Health: Regulation and Red Tape Reduction – The deregulation agenda – ARCHIVE
- Deregulation Unit within the Department’s Best Practice Regulation and Deregulation Division has been established to promote regulatory performance across the Health portfolio
March 19, 2014 – Abbott government as part the 2014 Autumn Repeal Day: The Australian Government Guide to Regulation – Cutting Red Tape, for the Australian Public Service involved in policy making, Honourable Josh Frydenberg MP, Parliamentary Secretary to the Prime Minister – READ, PDF, WEB
- It is no mandatory that every government policy proposal designed to introduce or abolish regulation must now be accompanied by an Australian Government Regulation Impact Statement (RIS)
- Australian Government has a plan to cut $1 billion in red tape every year. Regulation is “Any rule endorsed by government where there is an expectation of compliance”.
2013
International regulatory cooperation takes hold – “harmonisation” – regulatory assessment becomes consolidated
July 2013 – TGA International Engagement Strategy 2013-2015 (it begins) – Version 1.0 July 2013 – ARCHIVE, updated versions ARCHIVE, SOURCE
- “The therapeutic goods industry is becoming increasingly globalised and the TGA must be able to assure public confidence in products no matter where they are produced. As this is occurring, international regulators are demonstrating a greater willingness to develop partnerships and share information, skills and experience to ensure more informed and consistent decisions about the quality, safety and efficacy of therapeutic products.”
- “The major return on this investment is a reduction in duplication of effort in pre and post market evaluation of therapeutic goods, leading to a more efficient and effective regulatory system.” – i.e. to drive harmonisation
- The strategy will be reviewed periodically and amended as necessary in response to the changing international environment…
- The development of the joint Australia and New Zealand Therapeutic Products Agency (ANZTPA) [which eventually failed and was disbanded]
2011
May 31, 2011 – TGA – Biologicals legislation commenced following a recommendation from Commonwealth, State and Territory health ministers to improve the regulation of human tissues and cell-based therapies. – REF Providing the regulatory framework for biologicals
- What is regulated as a biological – Human derived – ARCHIVE
- Different to blood and tissued – ARCHIVE
- Don’d confuse “biological” with “biological medicine”, the latter is where “vaccine” is placed.
- First introduced in May 2011, the regulatory framework for biologicals provides the legislative basis for the regulation of human tissue and cell-derived products as well as live animal cell, tissues or organs that are supplied, in or exported from, Australia (TGA, 2017, 2021b). For those therapeutic goods, regulated by the TGA, and which are produced by genetic manipulation (GM), the TGA is required to seek advice from Office of the Gene Technology Regulator (OGTR) (TGA, 2004) – PDF
2010
November 1, 2010 – TGA introduced a streamlined submission process for prescription medicine applications that require the evaluation of non-clinical, clinical and/or bioequivalence information (category 1 and category 2 submissions). – REF
- Jan 17, 2011 – Transition information about the streamlined submission process – ARCHIVE
- To support sponsors in meeting the regulatory requirements under the streamlined submission process, a regulatory framework of legislative instruments has been created.
2009
The year the TGA introduced Business process reforms which included – AusPAR and PI/CMI
December 2009 – The first Australian Public Assessment Report (AusPAR) was published – a Publication of a regulatory decisions – REF, Australian Public Assessment Record (AusPAR) – ARCHIVE updated 2014 – READ
- The AusPAR project, part of the Prescription medicines business process reform project (BPR program) – “is increasing the transparency of the prescription medicine regulatory process by publicly releasing (since December 2009), Australian Public Assessment Records (AusPAR) for new products of major variations to existing products.” –REF
- “AusPAR provides information about the evaluation of a prescription medicine and the considerations that led the TGA to approve or not approve an application”
- “The TGA works with international counterparts in order to reduce the worldwide regulatory burden and increase the global uniformity of data requirements. Thus, wherever possible, the TGA’s requirements are the same as those of other major regulatory agencies.” [Thus FDA, EMA and UK medical agency important to take note of]
November 2009 – PI/CMI project launched – ARCHIVE
- “Since November 2009, the PI/CMI project provided via the TGA website “a single trusted source for product information (PI) and consumer medicine information (CMI)” to provide consumers and health professionals “enhanced access to prescription medicine information.” – REF,
April 2009 – The Industry Working Group (IWG) has provided advice and assistance on the range of business process reforms. Under the terms of reference, members of the working group are not representatives of individual sponsors, but bring specific expertise to the working group.” – REF
2009 – Streamlined Submission Process project (prescription medicines) “In early 2009, the TGA commenced a significant program of business process reforms (BPR program) for the regulation of prescription medicines in Australia” – REF
- “The key elements of the BPR program were identified during an industry consultation workshop held on 17 December 2007 which brought together representative of the TGA, Medicines Australia, and the Department of Health and Ageing. These initiatives are part of the Australian Government’s broader regulatory reform agenda.” – ARCHIVE
- There are three projects within the BPR program: –REF
2008
2008 – Department of Health and Ageing: Australian Health Management Plan for Pandemic Influenza (AHMPPI) 2008 – READ, REF,
- This plan was used the following year in the 2009 pandemic which ended up being “mild” – READ
2007
December 17, 2007 – TGA holds an industry consultation workshop – REF
- The key elements of the 2009 business process reforms (BPR program) “were identified during an industry consultation workshop held on 17 December 2007 which brought together representative of the TGA, Medicines Australia, and the Department of Health and Ageing.”
November 2007 – Biosecurity and Emergency Response: The National Health Security Act 2007 and the National Health Security Agreement – Dept Health 2007-08 Annual Report – PDF
- To improve the identification and response capacities of health authorities to national and international public health events, such as an influenza pandemic.
- N5H1 vaccines added to National Medical Stockpile
- Implemented outcomes of Oct 2006 National Pandemic Influenza Exercise — Exercise Cumpston’ 06 – READ
- Commenced revision of the Australian Health Management Plan for Pandemic Influenza (2003) to ensure that the plan is based on the latest evidence. (See 2008)
2006
October 2006 – Therapeutic Goods Administration and Biologics and Genetic Therapies Directorate (BGTD) of Health Canada – Parallel Review Pilot Project – ARCHIVE
- The project will involve the two agencies reviewing one or more submissions in parallel with an aim to enhance cooperation between regulators in various jurisdictions around the world.”
- November 8, 2006 – Health Canada:
October 16-19, 2006 – Exercise Cumpston’ 06 : Australia holds Australia’s largest ever health exercise and was one of the first major exercises on pandemic influenza conducted in any country – REPORT, READ, TIMELINE
July 10, 2006 – “On July 10, 2006, Health Canada issued a Notice of Compliance to Merck Frosst Canada Ltd. for the vaccine product GardasilTM – ARCHIVE
2005
2005 – “Australia had been in the pandemic “ALERT” phase since 2005 following the emergence of the avian influenza A(H5N1) virus infection in humans.” REF
2004
August 19-20, 2004 – The 9th National Public Health Association of Australia Inc (PHAA) Immunisation & 1st PHAA Asia-Pacific Vaccine Preventable Diseases Conference: ‘Immunisation at the crossroads: Challenges and Strategies‘ –READ, REF, PHA is an advocacy group – ARCHIVE
- PHAA Vaccines in Public Health Short Course before the conference!
- 1998 the 6th conference Immunisation Beyond 2000 – HERE
January 2004 – Dept Health & Ageing: Protecting Australia against Communicable Disease: Everybody’s Business – PDF (Referenced by Halton)
- “Influenza virus is a continuing global threat because it can spread easily and evolve rapidly to elude our prevention and treatment strategies.” [i.e. flu vaccines]
2003
November 5, 2003 – WHO Global Training Network (GTN) course on vaccine regulation for National Control Authorities – ARCHIVE
- “The Therapeutic Goods Administration (TGA) is one of the accredited training centres around the world that contributes to the WHO Global Training Network (GTN). The GTN was developed in 1996 as a means of providing educational resources to staff of vaccine production facilities and regulatory agencies responsible for control of vaccines.”
October 2003 – The Australian Action Plan for Pandemic Influenza was endorsed by the National Public Health Partnership and the Australian Health Ministers’ Advisory Council – Dept Health Annual Report 2003-04 – by Secretary of Department of Health and Ageing, Jane Halton – ARCHIVE, REF
- The 2003-04 outbreak of avian influenza H5N1 (bird ’flu) in Asia “was the second time in as many years that an unknown and highly contagious disease had posed a major international threat, the previous one being SARS” (a coronavirus)
- So Australia compiles an influenza plan (not a respiratory virus pandemic plan) to provide direction for the Australian, State and Territory governments and emergency services in the event of an influenza pandemic.
- “Influenza pandemics result from a major change in the structure of the influenza virus and are expected to occur about every 30 years. Experts predict that future pandemics are likely, if not inevitable.”
- [They got their wish 6 years later, following the May 2009 definition change for what constitutes a pandemic, except there was pushback in Europe
- “The expectation of 50 years ago that humanity would soon wipe out infectious disease, has been replaced with the knowledge that we will probably never win the ‘arms race’ against ever-changing microbes and viruses” writes Jane Halton [1953 Salk polio vaccine was released], now in makes sense why she headed the organisation that would save the world with 100-day vaccines for Disease X!
- Jane Halton became head of CEPI when it launched in 2017, in time for 2020 pandemic!
June 4, 2003 – TGA Laboratories (TGAL) are designated by the World Health Organization (WHO) as a WHO Collaborating Centre – ARCHIVE, ARCHIVE2
- WHO Collaborating Centre for Quality Assurance of Vaccines and other Biologicals and
- WHO Collaborating Centre for Drug Quality Assurance
- WHO Vaccine Self Sufficiency Program in the Western Pacific Region – REF
2002
January 29, 2002 – TGA Media Release: TGA sweeps Internet for false claims – ARCHIVE
- Australian Internet Sweep Day, coordinated by the Australian Competition and Consumer Commission (ACCC), is part of an international evaluation of web sites, aimed at protecting consumers and promoting fair trading in the global market. Linked through the International Marketing Service Network (IMSN) of of consumer protection authorities.
May 2002 – NCIRS: Vaccine preventable diseases and vaccination coverage in Australia 1999 to 2000 – PDF, SOURCE, CREDIT
2000
November 26-28, 2002 – WHO/IVR: Ethical considerations arising from vaccine trials conducted in paediatric populations with high disease burden in developing countries – PDF
- “Each year, millions of children in developing countries suffer from infectious diseases. Of these, more than 2.7 million children under five (WHO 2001 estimate) die from diseases that are potentially preventable by vaccines. In light of these unacceptable rates of morbidity and mortality, the development or improvement of vaccines to meet the needs of children in developing countries continues to be one of the highest priorities, with the attendant requirement for an increasing number of trials to evaluate new vaccines.”
- [This is an important claim which is parroted by WHO yet supply without citation]
- A cautious approach is appropriate in the conduct of vaccine trials among children in any circumstances because of their particular vulnerability in view of their inability to give informed consent and their sometimes greater potential to adverse reaction to vaccines.”
July 14, 2000 – Australian Children’s Hospital at Westmead and Children’s Medical Research Institute joint initiative: Gene Therapy Research Unit – ARCHIVE, REF
1998
January 1998 – TGA commenced an orphan drug program – ARCHIVE, PDF
- The “program aimed at encouraging sponsors of prescription medicines for treatment of rare diseases to register and market these medicines in Australia. Because these medicines are for a very limited number of patients, they are less likely to be commercially attractive to sponsors than medicines with a larger market. If the sponsor can substantiate that a medicine meets orphan drug criteria, the TGA will waive the cost of evaluating the medicine.”
- [Think ivermectin for COVID-19!]
1997
1997 – National Centre for Immunisation Research and Surveillance (NCIRS) of Vaccine Preventable Diseases was established by the National Centre for Disease Control, Commonwealth Department of Health and Aged Care as an initiative under the Seven Point Plan for the “Immunise Australia” program. – (lots of ref) ARCHIVE, Research – ARCHIVES, Vaccine trials – ARCHIVE
- NCIRS has provided editorial and scientific support for the production of the Australian Immunisation Handbook and provided support to the Australian Technical Advisory Group on Immunisation (ATAGI)
- NCIRS 2002 report: Vaccine preventable diseases and vaccination coverage in Australia, 1999 to 2000 – PDF
- NCIRS 2001 edition: Immunisation – Myths & Realities. Responding to arguments against immunisation – a guide for providers (first pub. October 1994) – PDF
- Look at charts on pg 10 & 11, and compare the dates on the Y-axis to comparable graphs found, and the Y-axis units – HERE, this is how they “debunk”.
February 1997 – Australian Government Immunise Australia Program: Population Health Division: The Seven Point Plan – ARCHIVE
- The Seven Point Plan was approved by Cabinet and launched by the Minister for Health and Family Services, the Hon Dr Michael Wooldridge, in February 1997
- Initiatives (incentives) for Parents.
- A bigger role for General Practitioners.
- Monitoring & Evaluation of Immunisation Targets.
- Immunisation Days.
- Measles Eradication.
- Education and Research.
- School Entry Requirements
1995
October 1, 1995 – Therapeutic Goods Regulations – Definitions antiseptic, fungicide, viricide etc added – PDF, READ
1992
August 18, 1999 – Therapeutic Goods Administration Laboratories Branch – The TGA laboratories (TGL)were first occupied in the spring of 1992. – REF, ARCHIVE
- “TGAL is responsible for evaluating the chemistry, quality control and manufacturing data submitted by the manufacturer in support of: vaccines, cytokines, blood products, hormones and enzymes, other biological medicines, monoclonal antibodies”
- Laboratory Information Bulletin Vol. 6, no. 1 (September 1994) – ARCHIVE, SOURCE
1989
1989 – The Therapeutic Goods Act 1989 came into effect in 1991 giving the Commonwealth more clearly defined regulatory authority. – READ Followed by the Therapeutic Goods Regulations 1990 – READ
- The Therapeutics Goods Administration (TGA) was created as a Division of the (then) Department of Community Services and Health – history – READ
- The legislation was to streamline Commonwealth and State legislation and to allow the Government to charge fees to industry to meet the costs of therapeutic goods regulation – REF
1983
1983 – A New Pandemic emerges HIV/AIDS- Much of the world’s optimism about the state of health was shattered by a new, mysterious pandemic, HIV/AIDS. In Australia, the virus was first identified in a patient in Sydney in 1983 – REF
1981
1981 – WHO: World Health Day with the ‘Health for all by the year 2000‘ – REF
1966
1966 – Therapeutic Goods Act (1966) – READ, CREDIT
- Therapeutic Goods Regulations (1970) – READ
- This Act and Regulations held until the big overhaul happened in 1989.
1953
1953 – Therapeutics Substances Act (1953) passed – READ, CREDIT
- The regulations were not enacted until 1956 , after the polio vaccine Cutter incident debacle in the USA – READ
1937
1937 – The Federal Health Council (1925) is expanded to become the National Health and Medical Research Council (NHMRC) with State and Federal representatives, now to include members of the medical establishment – REF
1937 – The Commonwealth Government passed a Therapeutic Substances Act in 1937, giving the Minister for
Health power to control the importation and exportation of substances which were declared in the Gazette
to be therapeutic substances. – (more history) – REF
1936
1936 – Therapeutics Substances Act (1936) signed, followed then by 1937, neither Act were proclaimed because of WWII – TGA History – REF
1926
1926 – Australia hosted the first League of Nations’ international conference on health – REF
- The delegates discussed early warning systems for epidemics and quarantine issues, as well as various medical problems… then the Great Depression hit followed by World War II, after which the World Health Organisation is formed, under the umbrella of the United Nations
1925
1925 – Federal Health Council to promote Federal-State cooperation established following a Royal Commission Commonwealth health system – REF
1921
March 1921 – The Commonwealth Department of Health was established – ARCHIVE
- “The panicked response to the 1919 influenza pandemic was a catalyst for founding the Commonwealth Department of Health in March 1921 .
- Other factors included recommendations from the State Premiers, the British Medical Association of Australia (the forerunner of the Australian Medical Association) and a blunt appeal to the Prime Minister, Billy Hughes, by Dr Victor Heiser from the Rockefeller Foundation’s International Health Board. Heiser told Hughes that Australia would be considered backward until it acquired a national health department.
- Rockefeller Foundation provided the “Government the financial incentives of temporary expertise in industrial hygiene, public health engineering and administration, as well as training scholarships” – REF
- Billy Hughes would go on to become Minister for Health and Repatriation – REF
–
1919
1919 – “Spanish influenza” was brought to Australia by returning servicemen. “The epidemic killed 12,000 Australians. It caused widespread panic. Troops were quarantined before being reunited with their families. Public meeting places such as hotels and theatres were closed, and masks had to be worn on the streets. The authorities’
response to the crisis was chaotic, with police being stationed along State borders to try to prevent the disease from spreading. – REF, HISTORY
- The panicked response to the 1919 influenza pandemic was a catalyst for founding the Commonwealth Department of Health in March 1921 – REF
1919 – The first international flight to Australia by Ross and Keith Smith, pointed to the need for improved quarantine and health services – REF
1916
1916 – Australia work with the Rockefeller-funded International Health Board to extend its hookworm eradication campaign to Australia – REF
1916 – WWI prompted the establishment in 1916 of the government Commonwealth Serum Laboratories to provide a source of anti-toxins and other biological agents, should overseas, supplies become scarce… – REF
1909
1909 – The Federal Quarantine Service within the Department of Trade and Customs is established – REF
- Commonwealth quarantine officers became part of an informal network of health officials and doctors operating at all levels around the country. It’s members started the public health movement leading to the Department of Health
- In 1900, life expectancy for men was 52 and 55 for women – REF
- In 1984 the quarantine responsibilities of the Department of Health,which was once its very nucleus, were transferred to the Department of Primary Industry – REF
1901
January 1, 1901 – Birth of the Commonwealth of Australia – TIMELINE





