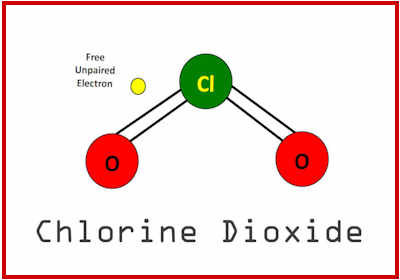
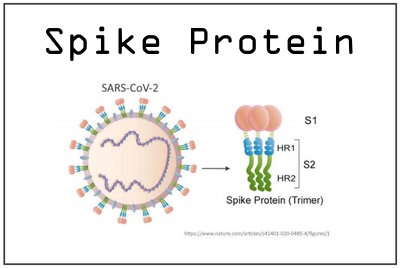
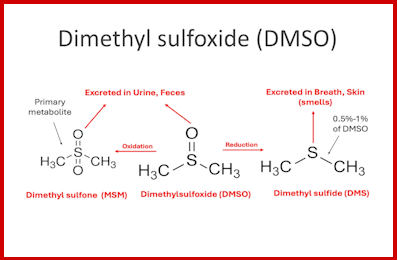
IF early treatment therapeutics were “found” to be “effective” in preventing hospitalisations and death from COVID-19, then not only would the pandemic have been over by 2020, the one-size-fits-all vaccine narrative would have been quashed.
Authorities could not legally justify mandating any experimental, emergency use product on its citizens, if an alternative, already registered drug product or nutraceutical proved successful in preventing death. Information about the effectiveness of off-patent drugs against the “novel” virus (and other viruses) is a massive threat to the multi-billion dollar pharmaceutical-industrial complex – especially the “vaccine” arm – which is their most profitable products – thanks to their liability shield.
Off-patent drugs, repurposed for off-label use
Existing, registered drugs have an already established, dose-related, potential side-effect profile for safety. Repurposing drugs, based on scientific literature and anecdotal evidence and experience is legal, and known as off-label use. Meaning the drug can be used with in registered dose on a dis-ease or pathogen which is not written on the product’s label – at the doctor’s discretion.
‘Off-label’ prescribing occurs when a drug is prescribed for an indication, a route of administration, or a patient group that is not included in the approved product information document for that drug.
2013
Once a drug patent expires, generic manufactures can legally make and supply that drug, which brings the price down dramatically, as such the drug is no longer highly profitable to the founding company, and they have no financial incentive to do trials to add extra indications to the label.
Since early 2020 frontline doctors around the world have been successfully using existing drugs, which happen to be off-patent, to treat patients diagnosed with COVID-19. Public Health agencies such as America’s NIH, who funded Randomised Controlled Trials (RTC) for these off-patent drugs, most often designed the trials to fail – be it timing of the drug or the dose administered, or both.
New, Patented drugs
On the other hand expensive, novel, new drugs that were still under patent, did happen to get emergency use approval, based on trial designs that were less stringent, and openly manipulated after the trial began. The patented drugs have already established concerning side effects, and long-term safety may not be known.
This page contains links to the therapeutics used during the COVID-19 pandemic, and scroll down for treatment protocols for Vaccine injury.
THis website is for informational purposes only – a historical record of events.
Nothing on this website should be seen or thought to represent medical Advice.
Early COVID-19 Treatments:
“Acceptable” patented COVID-19 Drugs:
Evolving protocols for vaccine injury