The definition of “Gene Therapy” appears to mean different things to different bodies. This page is an attempt to log definitions and understand the evolution of “gene therapy” from the context of how designated “gene therapy” products (i.e. Moderna and BioNTech) could suddenly be accepted as a “vaccine”.
There are established regulatory pathways for “vaccines” but not for “gene therapies”. In 2020 a gene therapy technology that encoded an “antigen protein” was accepted as a “vaccine”. If the only change to that product (now considered a platform technology) was to change the mRNA code to one that encodes a human protein, would it still be accepted as a “vaccine”?
Other pages on this website with additional information and context –
- Australia’s Gene Technology Regulator (OGTR) – HERE
- Australia’s OGTR did NOT assess the new gene technology vaccines – HERE
- Australia’s TGA and Regulation Timeline – HERE
- COVID-19 enquiries – HERE
- Revelations from the Pfizer FOIA Documents – HERE, and Moderna FOIA docs – HERE
- Lipid Nanoparticle (LNP) Technology (gene therapy delivery vehicle, for gene transfer) – HERE
- Can “vaccine” modified RNA impact human DNA? – HERE
Links in reverse chronological order
This page is continuously be updated as new data points come to my attention
2024
April 11, 2024 – Report 98: FDA Selected Its ‘Vaccines Advisory Committee’ – Not Its Gene Therapy Advisory Committee – to Recommend the COVID Injections for Emergency Use, to Hide the Fact that the Products Are Not Vaccines But Gene Therapies – READ includes notes from VRBPAC meetings
- “Why Did the CDC Change the Definition of ‘Vaccine’ and ‘Vaccination’ in September 2021, 10 Months After EUA Was Granted to Pfizer and Moderna for Their COVID-19 Drugs?…Was this for commercial reasons?
- “The Pfizer and Moderna COVID-19 drug products were labelled as “vaccines” well before the “vaccine” definition changed, and the FDA assigned VRBPAC as the advisory committee reviewing the COVID “vaccines”-related data as early as May of 2020. But, are these COVID drugs truly “vaccines,” or are they gene therapies, as defined by the FDA? The distinction matters since it drives the review and approval processes for the drugs.” See Table 1: VRBPAC or CTGTAC?
- “This improper categorization of the drugs as “vaccines” rather than gene therapies, provided the public a false sense of security that they would be protected from illness, when in fact the modified mRNA drugs were built on a novel platform with no long-term safety or efficacy record.”…The gene therapy testing and approval process is decidedly more demanding”
- FDA’s guidance regulations for vaccines…vaccine products “shall meet generally accepted standards of purity and quality.” versus Gene Therapy products “only product lots that meet defined specifications or acceptance criteria are administered during all phases of clinical investigation and following market approvals.” In “FDA’s own words, that gene therapy products undergo a much more thorough level of scrutiny.”
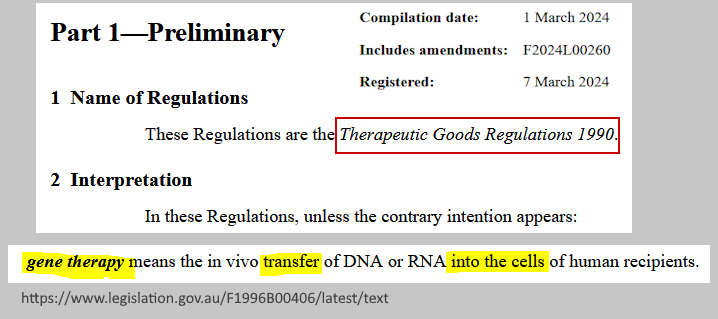
March 7, 2024 – Therapeutic Goods Regulations (1990) – definition of “gene therapy” – READ, PDF
- “gene therapy means the in vivo transfer of DNA or RNA into the cells of human recipients”
(nothing is stated about integrations into DNA) – source, PDF
January 17, 2024 – The European lobby Kangaroo Group, hosted a lunch in the European Parliament with Members with BioNTech and Moderna as key speakers, to discuss the forthcoming revision of “European Commission’s proposal for a reform of the EU General Pharmaceutical Legislation (GPL)”. EU- ARCHIVE, KG – ARCHIVE, PDF, CREDIT
- Their objective to effectively change the definition of gene therapy, under which their COVID-19 mRNA vaccines are classified, and to create a “definition of Platform Technologies, as well as a clear demarcation between Gene Therapies Medicinal Products that alter human genomes, and those that do not (e.g. mRNA).”
January, 2024 – FDA: Guidance for Industry: Human Gene Therapy Products Incorporating Human Genome Editing –PDF (updated!), ARCHIVES, Jessica Rose – CREDIT GE = genome editing
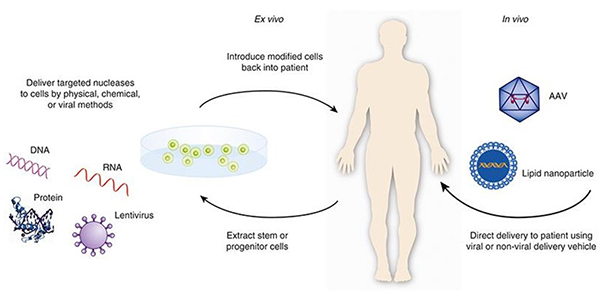
- “Viral vectors may support sustained expression of GE component transgenes, and nanoparticles may allow the temporal delivery of GE components as DNA, RNA, or proteins” [lipid nanoparticles can replace the virus vector!]
- “When administered in vivo in the form of DNA, RNA and/or protein via nanoparticles, the GE components are considered the active pharmaceutical ingredients or drug substances.” i.e. the genome editing materials are for the sake of the regulator considered the active ingredient!
- The SV40 promoter contaminant is also a GE component of the Pfizer mRNA injectable products
- “Assessment of bio-distribution should be conducted to characterize the distribution, persistence, and clearance of the GE product, any expressed GE components in vivo, editing activity in target and non-target tissues, and the potential for inadvertent germline modification.” [they skipped this step with the mRNA injectable products]
2023
January 12, 2023 – Full Fact: Andrew Bridgen wrong to call mRNA vaccines gene therapy – READ
January 6, 2023 – Fierce BioTech: Pfizer pivots from early-stage rare disease R&D, shifting to external innovation and putting assets up for sale – READ
- They will “pullback from new viral-based gene therapies and early-stage rare disease work in general”. [so much for being a gene-therapy leader announced in 2016 – REF], now wanting to be “best positioned to generate high-impact medicines and vaccines.” [of course they do!]
2022
July 4, 2022 – TGA Advanced Therapies: Report on ‘Cell, Gene and Tissue Regulatory Framework in Australia – A stakeholder engagement report on Advanced Therapies prepared for the Therapeutic Goods Administration – READ, Report – PDF, ARCHIVE
- In November 2021 the Therapeutic Goods Administration (TGA) commissioned MTP Connect to conduct a stakeholder review of the regulatory framework for gene, cell and tissue therapies in Australia.
- Medical Technologies and Pharmaceuticals Growth Centre (MTP Connect) “is Australia’s Life Sciences Innovation Accelerator – supporting Australia’s vibrant medical products sector…an independent, not-for-profit organisation established by the Australian Government to champion the continuing growth of Australia’s vibrant medical products sector” – WEB, Established November 2015 as part of the $248 million Industry Growth Centres Initiative – ARCHIVE, Factsheet –
- “Any biological or prescription medicine that involves genetic modification must also be approved by the Office of the Gene Technology Regulator (OGTR). OGTR approval is needed before TGA approval… ” REF
- [Yet COVID-19 mRNA “vaccines” and their approved “platform”, which are genetically modified, gene therapy, biological medicines escaped this oversight!]
- European Medicines Agency (EMA) guidance
- Reference to FDA – Cellular & Gene Therapy Products which is regulated by Center for Biologics Evaluation and Research (CBER) including vaccines – READ, ARCHIVE
- MTP Connect: mRNA Lecture Series 2: Lecture Three – Preparing for Disease X , speakers Moderna, WHO and Doherty institute [Moderna’s factory is being built in Mebourne!] – READ, ARCHIVE
- First lecture series “mRNA Victoria” – READ, ARCHIVE, WEB
- Monash RNA – home to Australia’s largest network of RNA and mRNA researchers. – WEB
- August 9, 2022 – Victorian mRNA Innovation Hub (VMIH) – $5.4M – VMIH is a world-first collaboration between Monash University, the University of Melbourne, Doherty Institute and Monash Institute of Pharmaceutical Sciences to develop next-generation vaccines and therapeutics to treat a range of diseases. – REF, WEB
- The funding, which is part of the state government’s mRNA Victoria Activation Program initiative aims to develop new technologies that will underpin mRNA therapeutics and vaccines that are more effective, cheaper and faster to produce.
May 10, 2022 – Australian Dept Health: Is it true? Can COVID-19 vaccines alter my DNA? – ARCHIVE
- “The Pfizer/BioNTech COVID-19 vaccine uses a fragment of messenger RNA (mRNA) to instruct your body to make an immune response against COVID-19.” [Deceptive initial comment, and only 5 short sentences]
- [The mRNA instructs the body to produce a foreign protein, which is hoped that the body produces an immune response to the protein, not the mRNA. The hope is that mRNA does not elicit an immune response, which is why they changed uridine to pseudouridine]
- No mention of the DNA contamination in the “vaccine” vials!
March 3, 2022 – Dave Martin files lawsuit in Utah: lawsuit, Griner v. Biden et al in the U.S. District Court in Utah on behalf of Devan Griner, MD – READ, Uncover DC – PDF – READ, LISTEN, WATCH
- Dave Martin insists Insists the Injections are Gene Therapy Medical Devices – the body becomes a biological (weapons) factory, manufacturing foreign protein (in the case of an antigen code), the product does not stimulate any immunity, it is an instruction to make a “scheduled pathogen” – a genetic sequence derived from SARS coronavirus – WATCH
- Using the term “vaccination” was intended to mislead the public”, confuse and build expectation. It should have been called “gene therapy” as stated in the SEC filing in 2019 and 2020 – REF
- Moderna was set up as an outgrowth of 10 year National Science Foundation study on “gene therapy”
- In the The United States, since 1905, the Public Health Policy, says that the state public health agencies are entitles to do police powers on public health if they can disrupt infection or transmission. There is NO police power that has ever been granted, in any circumstance, for any reason, for a treatment.”
January 10, 2022 – Therapeutic Goods Administration (TGA): A NEW catagory has been added to TGA website called Advanced Therapies, under which gene therapies suddenly appears! – ARCHIVE, READ,
- Quitely added to the website with NO press release or statement – ARCHIVE, unable to find regulatory change around this time – READ
- Any biological or prescription medicine that involves genetic modification must also be approved by the Office of the Gene Technology Regulator (OGTR) [WOW! COVID-19 mRNA vaccines missed that step in 2020!]
- “We regulate therapies that involve in-vivo genetic manipulation of human cells as prescription medicines under section 23 of the Act. This includes small silencing RNAs, CRISPR and other gene editing technologies, and gene therapies administered by vectors.”
- Advanced Therapies defined : Advanced therapies or advanced therapy medical products is a term used to describe innovative therapies. International regulators use this term to include gene, cell and tissue therapies. – READ
- November 21, 2019 : Good Manufacturing Practice (GMP) for new and emerging technologies: Advanced Therapy Medicinal Products (ATMPs) – Presentation – ARCHIVE
2021
November 29, 2021 – World Health Summit 2021: Stefan Oelrich (Bayer): mRNA vaccines are Gene Therapy – WATCH, BACKUP, CREDIT, more VIDEOS
“Ultimately the mRNA vaccines are an example for that cell and gene therapy. I like to say that if we had surveyed two years ago, in the public, ‘Would you be willing to take gene or cell therapy and inject it into your body?’ we would have probably had a 95% refusal rate.”
Stephan Oelrich from Bayer at World Health Summit in 2021 (Nov 29, 2021)
August 10, 2021 – Reuters Fact Check: mRNA vaccines are distinct from gene therapy, which alters recipient’s genes – READ
January 4, 2021 – CDC: Bust Myths and Learn the Facts about COVID-19 Vaccines – COVID-19 vaccines do not alter DNA – ARCHIVE, all ARCHIVES, READ
Messenger RNA vaccines—also called mRNA vaccines—are the first COVID-19 vaccines authorized for use in the United States. mRNA vaccines teach our cells how to make a protein that triggers an immune response. The mRNA from a COVID-19 vaccine never enters the nucleus of the cell, which is where our DNA is kept. This means the mRNA cannot affect or interact with our DNA in any way. Instead, COVID-19 mRNA vaccines work with the body’s natural defenses to safely develop immunity to disease.
At the end of the process, our bodies have learned how to protect against future infection. That immune response and making antibodies is what protects us from getting infected if the real virus enters our bodies.
CDC: Will a COVID-19 vaccine alter my DNA?
2020
December 18, 2020 – CDC: Understanding mRNA COVID-19 Vaccines – New Approach to Vaccines – ARCHIVE [The plan all on one page – no mention of gene therapy though]
- “mRNA vaccines are a new type of vaccine to protect against infectious diseases.”
- “…That immune response, which produces antibodies, is what protects us from getting infected if the real virus enters our bodies.” [that failed!]
- “They do not affect or interact with our DNA in any way.”
- “mRNA Vaccines Are New, But Not Unknown…Researchers have been studying and working with mRNA vaccines for decades. Interest has grown in these vaccines because they can be developed in a laboratory using readily available materials. This means the process can be standardized and scaled up, making vaccine development faster than traditional methods of making vaccines.” [there you have it in a nut shell – the platform goal, taking a gene therapy model to vaccine]
- “mRNA vaccines have been studied before for flu, Zika, rabies, and cytomegalovirus (CMV)” – optative word “studied”, not fully assessed or ever before brought to market.
- “As soon as the necessary information about the virus that causes COVID-19 was available, scientists began designing the mRNA instructions for cells to build the unique spike protein into an mRNA vaccine.”
- “Future mRNA vaccine technology may allow for one vaccine to provide protection for multiple diseases, thus decreasing the number of shots needed for protection against common vaccine-preventable diseases.” [Frankenstein territory]
December 15, 2020 – GAVI: Will a mRNA vaccine alter my DNA? – ARCHIVE
June 30, 2020 – The FDA termed these COVID-19 mRNA drugs gene therapies. Moderna’s Q2 2020 quarterly report stated, “mRNA is considered a gene therapy product by the FDA…” – REF
January 12, 2020 – Moderna design mRNA “gene therapy” vaccine sequence in one hour, two days after the Chinese novel-CoV virus gene sequence was made public, and following discussions with their partner the NIH – TIMELINE
2019
November 21, 2019 : Good Manufacturing Practice (GMP) for new and emerging technologies: Advanced Therapy Medicinal Products (ATMPs) – Presentation – ARCHIVE, PDF
- Advanced Therapies which include gene, cell and tissue treatments was added to TGA industry Jan 10, 2022
August 21, 2019 – AskBio Applauds Pfizer’s Continuing Investments in Gene Therapy – READ, (see 2003 below)
- Pfizer announced a $500 million investment in a state-of-the-art gene therapy manufacturing facility that is based on Dr. Samulski’s and AskBio’s AAV technology discoveries.” Using adeno-associated virus (AAV) to deliver corrected genes to cells with genetic defects.
- Pfizer’s AAV therapeutic platform was made possible when they purchased Bamboo Therapeutics from AskBio in 2016 – READ
- The AAV groundbreaking gene therapy technology is the basis for treating a wide variety of diseases, including several diseases in AskBio’s clinical portfolio such as Duchenne Muscular Dystrophy (transferred to Pfizer as part of the Bamboo acquisition)
- 2024: AAV is driving today’s therapeutic discoveries and is used in the only two FDA-approved gene therapies currently available – READ, ARCHIVE
May 7, 2019 – Moderna Blog: Advancing the Frontiers of Our Platform Science – ARCHIVE
- “Today, we described our program of advancing new delivery technologies toward creating a generation of mRNA-based immune system therapeutics. This includes exciting translational work demonstrating that our novel immune nanoparticles are able to deliver mRNA to a significant proportion of T cells, Natural Killer cells, B cells and myeloid cells.We demonstrated that we can express functional proteins in vivo across multiple preclinical species, and ex vivo in human blood.” [sounds a lot like gene therapy]
- The latest science behind evading the innate immune system
March 13, 2019 – Moderna submitted their Form 10-K Annual Report to the Securities & Exchange Commission (SEC) – FDA recognises mRNA products as “gene therapy” and regulatory pathway uncertain – TIMELINE, IMG,
- “Currently, mRNA is considered a gene therapy product by the FDA“
- In that same filing they state “because no product in which mRNA is the primary active ingredient has been approved, the regulatory pathway for approval is uncertain. The number and design of the clinical and preclinical studies required for the approval of these types of medicines have not been established…”
2018
July 25, 2018 – FDA: What is Gene Therapy? – ARCHIVE, READ
- July 2018 – Draft Guidance for Industry: Long Term Follow-Up After Administration of Human Gene Therapy Products – PDF
- “Gene therapy is a technique that modifies a person’s genes to treat or cure disease.” As noted in the diagram below the direct delivery of RNA can be non-viral such as lipid nanoparticles
- “Clinical studies in humans require the submission of an investigational new drug application (IND) prior to initiating clinical studies in the United States.”
- So what are the IND rules? (July 20, 2022) – READ
2017
December 14, 2017 – PRESS RELEASE: Asklepios BioPharmaceutical, Inc. Launches New Portfolio Company – Actus Therapeutics, Inc. – READ, ARCHIVE (enzyme replacement therapy), Actus website – ARCHIVE
- Feb 1, 2017 – Scientists ready to test gene therapy for Pompe disease “…gene therapy designed by Duke University researchers to improve treatment for the genetic disorder Pompe disease is set to enter a Phase 1 clinical trial” – READ, STUDY, REF
November 15, 2017 – Moderna’s first in-human Phase 1 “cancer vaccine” clinical trial – TIMELINE
2016
November 29, 2016 – AGTC: Gene Therapy Explained – how gene therapy, delivered by AAV vectors, works – WATCH, AGTC is a startup company – WATCH,
- Applied Genetic Technologies Corporation (AGTC) was founded by five scientific leaders in the use of viral vectors for Gene Therapy and began operations in 2001 – REF, AGTC website dates back to 1996 – ARCHIVE, and was founded 5 years earlier ~1991 – REF ,begining with DNA/RNA lab tool – REF
August 1, 2016 – Pfizer: Pfizer aims to become industry leader in Gene Therapy with Aquisiton of Bamboo Therapeutics Inc – ARCHIVE, Pfizer gene therapy – WEB
- Jude Samulski, Chief Scientific Officer and Executive Chairman of Bamboo Therapeuitics and a leading expert in the field of rAAV vectors with more than 25 years of experience, will be joining Pfizer. Dr. Samulski, together with the Bamboo team, will play a key role in helping to develop and accelerate Pfizer’s capabilities in gene therapy.” (Dr R. Jude Samulski consults to the FDA on gene therapies, also see AskBio 2003 below)
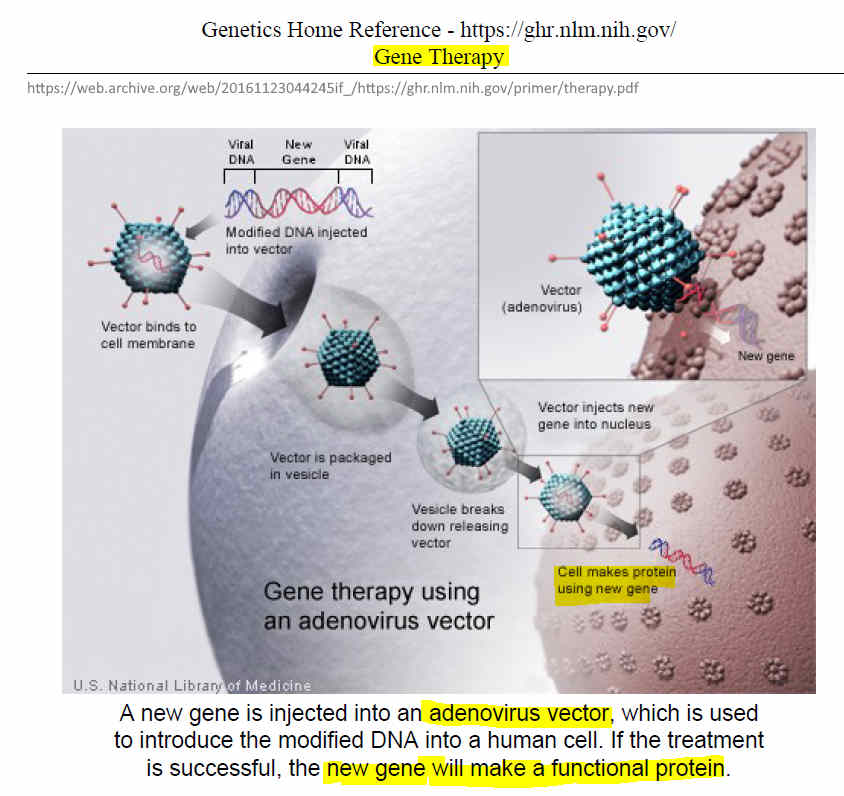
July 12, 2016 – NIH | Genetics Home Reference: What is gene therapy? (page launch) – ARCHIVE, PDF, CREDIT
- Several approaches to Gene therapy. Simply introducing the new gene into a patient’s cells. One key intent is to get the body to make protein.
“Gene therapy is designed to introduce genetic material into cells to compensate for abnormal genes or to make a beneficial protein.
What is gene therapy? – PDF, READ
- Some types of virus, such as retroviruses, integrate their genetic material (including the new gene) into a chromosome in the human cell. Other viruses, such as adenoviruses, introduce their DNA into the nucleus of the cell, but the DNA is not integrated into a chromosome”
- Adeno virus vector – just like Oxford/AstraZeneca’s “COVID-19 vaccine”
January 20, 2016 – NC biotech Center Press Relase: Bamboo Therapeutics Gearing Up to Treat Deadly Childhood Neurological Diseases – ARCHIVE, Bamboo Therapeutics website – ARCHIVE
- Bamboo Therapeutics – latest biotechnology company co-founded by entrepreneurs Sheila Mikhail and R. Jude Samulski, Ph.D., director of the Gene Therapy Center at the University of North Carolina at Chapel Hill – to treat devastating childhood neurological diseases with next-generation gene therapy – ARCHIVE, SOURCE, Bamboo is a spinout of Asklepios Biopharmaceutical (AskBio) a gene-delivery technology company co owned by Samulski et al.
- Bamboo has been operating as a virtual company in Chapel Hill with seed money from angel investors, foundations and the motivated parents… [22% stake or $43 million by Pfizer – REF]
- August 1, 2016 – Bamboo Therapeutics acquired by Pfizer for total of $645M – ARCHIVE, Pfizer Gene Therapy – READ
2015
2015 – TGA website: Acronyms & Glossary – Definitions – ARCHIVE, READ
- no definition for “Gene Therapy” on the web page yet it is defined in the TG Regulations!
2014
2014 – In 2014, Pfizer established within the company’s Rare Disease Research Unit the Genetic Medicines Institute (GMI) in London, UK, which is a dedicated gene therapy research group under the direction of leading gene therapy researcher Michael Linden. …Pfizer has research agreements with several leading academic institutions… –REF
2010
September 2010 – In September 2010, the Food and Drug Administration issued final regulations addressing the safety reporting requirements for investigational new drug applications (INDs) – REF
- “The emergency use of an unapproved investigational drug or biologic requires an IND…The need for an investigational drug or biologic may arise in an emergency situation that does not allow time for submission of an IND.” – REF
2009
May 28, 2009 – CDC: Cellular & Gene Therapy Products – first ARCHIVE
The Center for Biologics Evaluation and Research (CBER) regulates human gene therapy products – products that introduce genetic material into the body to replace faulty or missing genetic material, thus treating or curing a disease or abnormal medical condition.
- FDA has not yet approved any human gene therapy product for sale, but R&D is happening at a fast pace.
2006
March 29, 2006 – Uni North Carolina Chapel Hill | News Relese: First human clinical trial in the United States for gene therapy for muscular dystrophy now under way – ARCHIVE , Today AskBio has a divesture and license agreement with Pfizer – REF [This on post took me down a rabbit hole – Dr Samulski evolved into an FDA influencer,his journey started ~2000]
- The clinical trial for Duchenne muscular dystrophy (DMD) tests the safety and effectiveness of a therapy that was developed over two decades by scientists at the University of North Carolina at Chapel Hill’s School of Medicine and the University of Pittsburgh.
- In the trial, six boys with DMD will receive replacement genes for an essential muscle protein. Each of the boys will receive replacement genes via injection into a bicep of one arm and a placebo in the other arm.
- “The therapy was made possible by the pioneering research in [adeno-associated virus] AAV by Dr. Richard Jude Samulski, professor of pharmacology and director of the Gene Therapy Center at UNC…”
- Using new BiostrophinTM therapy – by Asklepios BioPharmaceuticals, Inc. (AskBio) is cross licensed with GlaxoSmithKline, …AskBio will gain exclusive access to selected recombinant adeno-associated virus vector (“rAAV”) serotypes that GSK licensed from the University of Pennsylvania – REF, Biostrophin product claimed launched 2010 – REF, but not in their news? – HERE
- AskBio is a private development-stage biotechnology company, which was spun out of the University of North Carolina – Chapel Hill.
- [curously University of Pennsylvania (UPenn) owns an Aug 2006 patent for mRNA pseudouridine with Weissman and Karikó – TIMELINE, READ, then worked with Acuitas Therapeutics’ LNP in Nov 2014 ]
- Jan 7, 2021 – AskBio Applauds Commencement of Pfizer’s Pivotal Trial for Duchenne Muscular Dystrophy – Phase 3 trail begins with new drug candidate PF-06939926 in CIFFREO trial [seems Biostrophin didn’t work out] – READ
- “Dr. Samulski is a former member of the Recombinant DNA Advisory Committee (RAC), a committee tasked with assisting the FDA with approving or disapproving gene therapy clinical trials in the United States. Dr. Samulski also frequently serves as a gene therapy consultant to the FDA. In 2008, Dr. Samulski was recognized by the American Society of Cell and Gene Therapy with the Inaugural Lifetime Achievement Award for his work.” – REF
2004
July 9, 2004 – US Department of Energy: Human Genome Project – “Gene Therapy” – ARCHIVE
- “In most gene therapy studies, a “normal” gene is inserted into the genome to replace an “abnormal,” disease-causing gene. A carrier molecule called a vector must be used to deliver the therapeutic gene to the patient’s target cells. Currently, the most common vector is a virus that has been genetically altered to carry normal human DNA. Viruses have evolved a way of encapsulating and delivering their genes to human cells in a pathogenic manner. Scientists have tried to take advantage of this capability and manipulate the virus genome to remove disease-causing genes and insert therapeutic genes.”
- “Another nonviral approach involves the creation of an artificial lipid sphere with an aqueous core. This liposome, which carries the therapeutic DNA, is capable of passing the DNA through the target cell’s membrane.”
- “The Food and Drug Administration (FDA) has not yet approved any human gene therapy product for sale. Current gene therapy is experimental and has not proven very successful in clinical trials. Little progress has been made since the first gene therapy clinical trial began in 1990. In 1999, gene therapy suffered a major setback with the death of 18-year-old Jesse Gelsinger….”
- “”Another major blow came in January 2003, when the FDA placed a temporary halt on all gene therapy trials using retroviral vectors in blood stem cells. FDA took this action after it learned that a second child treated in a French gene therapy trial had developed a leukemia-like condition…”
- Limitations of gene therapy includes:
- “Immune response – Anytime a foreign object is introduced into human tissues, the immune system is designed to attack the invader. The risk of stimulating the immune system in a way that reduces gene therapy effectiveness is always a potential risk. Furthermore, the immune system’s enhanced response to invaders it has seen before makes it difficult for gene therapy to be repeated in patients”.
- Also Short-lived nature of gene therapy, Prombles with viral vectors and multigene disorders
2003
2003 – Asklepios Biopharmaceutical Inc. (AskBio) is a private development-stage biotechnology company, which was spun out of the University of North Carolina – Chapel Hill (UNC) Biotech Centre. – ARCHIVE, website ARCHIVES
- AskBio is “engaged in the development and delivery of novel protein and cellular based therapies through design of proprietary Biological Nano ParticlesTM (BNP)” technology platform – ARCHIVE, which will become known as adeno-associated virus (AAV) – READ
- By 2021 AskBio claim they started in 2001,and are a subsidiary of Bayer AG acquired in 2020, and Pfizer launches first Phase 3 trial for DMD – REF
February 14, 2003 – Dolly the Sheep, the first cloned mammal, is euthanised at age 6 (1996-2003) – READ
- “Dolly, the first mammal to be cloned from adult DNA, was put down by lethal injection Feb. 14, 2003. Prior to her death, Dolly had been suffering from lung cancer and crippling arthritis. Although most Finn Dorset sheep live to be 11 to 12 years of age, postmortem examination of Dolly seemed to indicate that, other than her cancer and arthritis, she appeared to be quite normal. The unnamed sheep from which Dolly was cloned had died several years prior to her creation. Dolly was a mother to six lambs, bred the old-fashioned way.”
January 14, 2003 – FDA: FDA placed temporary halt on all gene therapy trials using retroviral vectors in blood stem cells – ARCHIVE, CREDIT
- The FDA’s Biological Response Modifiers Advisory Committee (BRMAC) – “took this action after it learned that a second child treated in a French gene therapy trial had developed a leukemia-like condition.” Treated for “bubble baby syndrome.”
2002
May 12, 2002 – New Scientist: DNA nanoballs boost gene therapy – ARCHIVE, CREDIT – nano particles enter nucleus!
- “Scrunching up DNA into ultra-tiny balls could be the key to making gene therapy safer and more efficient. The technique is now being tested on people with cystic fibrosis.”
- Researchers at Case Western Reserve University and Copernicus Therapeutics are able to create tiny liposomes 25 nanometers across small enough to enter the nuclear pores and carry therapeutic DNA through pores in the nuclear membrane!
- “The nanoparticles consist of a single DNA molecule encased in positively charged peptides and are themselves delivered to cells via liposomes. In cells grown in culture, there was a 6000-fold increase in the expression of a gene packaged this way compared with unpackaged DNA in liposomes.”
- [Now think short length DNA contamination wrapped in LNP in Pfizer’s mRNA COVID-19 “vaccine” – does that have the potential to get into the cell nucleus and incorporate into chromosomes?]
2000
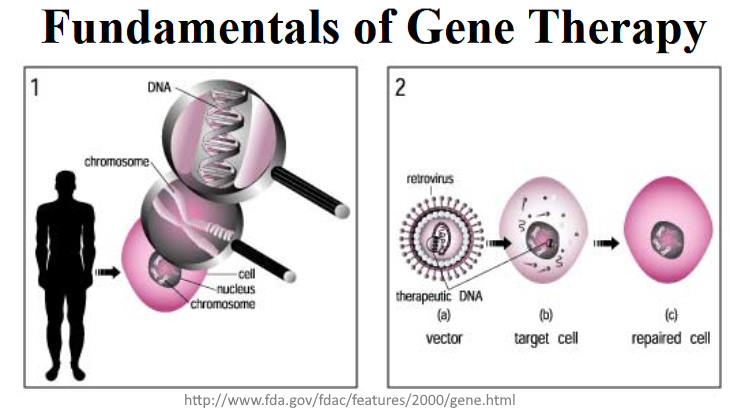
September 2000 – FDA: Fundamentals of Gene Therapy – ARCHIVE, FDA Consumer Magazine – SOURCE
- “DNA, carry the information needed to make proteins, the building blocks of our bodies. The body buries genes deep in the heart of every cell, the nucleus, and organizes them in the chromosomes that hold the DNA. But when your DNA is damaged, it no longer makes all the needed proteins and disease results….”
- “To reverse disease caused by genetic damage, researchers isolate normal DNA and package it into a vector, a molecular delivery truck usually made from a disabled virus. Doctors then infect a target cell —usually from a tissue affected by the illness, such as liver or lung cells—with the vector. The vector unloads its DNA cargo, which then begins producing the missing protein and restores the cell to normal.”
September 2000 – Human Gene Therapy and The Role of the Food and Drug Administraion – ARCHIVE, TIMELINE
- FDA’s Center for Biologics Evaluation and Research (CBER) began to regulate the emerging market of human gene therapy products, which fall under the legal definition of a “biologic”
- It was reported that since 1989 to September 2000, the FDA had “received about 300 requests from medical researchers and manufacturers to study gene therapy and to develop gene therapy products” – purpose to find cures for genetic diseases.
July 14, 2000 – Australian Children’s Hospital at Westmead and Children’s Medical Research Institute joint initiative: Gene Therapy Research Unit – ARCHIVE, REF
1999
December 15, 1999 – NIH | Secretary of Health and Human Services | Secretary’s Advisory Committee on Xenotransplantation (SACX) – ARCHIVE, SACX – ARCHIVE, with in the Office of Biotechnology Activities
November 19, 1999 – NIH | Office of Biotechnology Activities: Recombinant DNA and Human Gene Transfer – ARCHIVE, ARCHIVE
1998
March 1998 – HHS | FDA: Guidance for Industry – Guidance for Human Somatic Cell Therapy and Gene Therapy – PDF
- “Recently, various innovative therapies involving the ex vivo manipulation and subsequent reintroduction of somatic cells into humans have been used or proposed”…
- “Gene therapy is a medical intervention based on modification of the genetic material of living cells. Cells may be modified ex vivo for subsequent administration to humans, or may be altered in vivo by gene therapy given directly to the subject.”
1996
July 5, 1996 – Roslin Institute, Edinburgh: Dolly the Sheep is born, marking the first mammal to be cloned from adult DNA – ARCHIVE, CREDIT, WIKI, Roslin Institute – CLONING – READ Dolly is euthanised in Feb 2003
- “In 1996, Roslin Institute and collaborators PPL Therapeutics created Dolly, the first animal cloned from a cell taken from an adult animal….Nuclear transfer is not a new technique. It was first used in 1952 to study early development in frogs and in the 1980’s the technique was used to clone cattle and sheep using cells taken directly from early embryos.” – REF
- PPL Therapeutics is a world leader in the application of transgenic technology to the production of human proteins for therapeutic and nutritional use.” – ARCHIVE, The web domain discontinued by 2005 – ARCHIVES
- Dolly was mated Nov 1997 and “had a little lamb” called Bonnie – ARCHIVE
1996 – University of North Carolina (UNC) at Chapel Hill: The University of North Carolina School of Medicine created the Gene Therapy Center in 1996 with the goal of merging molecular genetics research with healthcare delivery. The facilities comprise the Vector Core (est 1994) and the Human Applications Laboratories ,- ARCHIVE, WEBSITE
- Dr. Richard Jude Samulski, professor of pharmacology became the first director of Gene Therapy Center– REF
- Jude Samulski and team pioneered the research & developed of a virus known as adeno-associated virus (AAV), and in 1996 published a report of the first muscle gene delivery involving an AAV vector.
- “Samulski has long pioneered methodologies for making viruses deliver genes. As a graduate student at the University of Florida in the early 1980s, his thesis project was developing the AAV as a vector for therapeutic genes.” In 1986 he moved to University of Pittsburgh and his first graduate student was Xiao Xiao, who had just come from China.
- “Xiao and Samulski put their projects together and formed Asklepios BioPharmaceutical Inc. (AskBio) in 2003. Along with the rights granted by UNC to Samulski’s AAV vector technology, AskBio acquired the intellectual property rights to Xiao’s uniquely miniaturized dystrophin gene
Gene transfer can be broadly defined as the introduction of new “foreign” genes, usually in the form of DNA, into the individual cells of the body.
If a normal copy of the defective gene can be transferred into a cell, it is anticipated that the normal protein product derived from the “new” gene would supply the missing function in a cell and consequently cure the cellular dysfunction.
UNC Gene Therapy Centre
1995
July 11-12, 1996 – FDA & NIH: FORUM 1996: GENE THERAPY – PDF, SOURCE

1993
October 14, 1993 – HHS | FDA: Federal Register: Application of Current Statutory Authorities to Human Somatic Cell Therapy Products and Gene Therapy Products – PDF, CREDIT
- The Food and Drug Administration (FDA) is making available, through this document, a statement of the manner in which FDA’s current statutory authorities governing therapeutic products apply to human somatic cell therapy products and gene therapy products.
Human gene therapy seeks to modify or manipulate the expression of a gene or to alter the biological properties of living cells for therapeutic use. FDA generally considers human gene therapy products to include all products that mediate their effects by transcription or translation of transferred genetic material, or by specifically altering host (human) genetic sequences.
Some examples of gene therapy products include nucleic acids, genetically modified microorganisms (e.g., viruses, bacteria, fungi), engineered site-specific nucleases used for human genome editing, and ex vivo genetically modified human cells…
- Additionally
Public Health Service Act (the PHS Act)… (42 U.S.C. 262(a)) identifies a biological product as “any virus, therapeutic serum, toxin, antitoxin, vaccine, blood, blood component or derivative, allergenic product, or analogous product, or arsphenamine or its derivatives (or any other trivalent organic arsenic compouod), applicable to the prevention, treatment, or cure of diseases or injuries of man.”
Licenses are to be issued upon a showing that the establishments and products “meet standards, designed to insure the continued safety, purity, and potency of such products * * *.”…A biological product’s effectiveness for its intended uses must be shown as part of the statutory requirement for potency (21 CFR600.3(s)).
- At the investigational stages, when the products are being studied in clinical trials to gather safety and
effectiveness data, biological products must meet the requirements… - The “safety” and “effectiveness” for a biological product i.e. vaccine, is (maybe only) assessed during clinical trials. Thus COVID-19 vaccines only had barely 2 months of “safety” assessment before unblinded – TIMELINE
- “Some products may contain a combination of biological products and drugs or devices. Under a provision of the Safe Medical Devices Act of 1990, FDA determines the primary mode of action of the combination products (21 U.S.C. 353(g)), then assigns the primary jurisdiction for review of the product within the agency based on that determination.”
- LNP/mRNA complex products are such combination chemical LNP + synthetic modified gene code (biological or chemical?)
Gene therapy products are defined for the purpose of this statement as products containing genetic material administered to modify or manipulate the expression of genetic material or to alter the biological properties of living cells.
Some gene therapy products (e.g., those containing viral vectors) to be administered to humans fall within the definition of biological products and are subject to the licensing provisions of the PHS Act, as well as to the drug provisions of the act. Other gene therapy products, such as chemically synthesized products, meet the drug definition but not the biological product definition and are regulated under the relevant provisions of the act only.
September 1993 – Janet Woodcock was the Director of FDA’s Office of Therapeutics Research and Review. – REF
- Why is this interesting? Woodcock was the FDA rep in 2020 that thwarted hydroxychloroquine and ivermectin’s use for COVID-19 early treatment, an act that allowed novel COVID-19 vaccines to be authorised under EUA. – Steven Hatfill EXCERPT, EXCERPT, EXCERPT
1993 – The Biotechnology Industry Organization (BIO) a biotech lobby group, is created through the merger of two existing organsation – ARCHIVE, TIMELINE
- The merger of the Industrial Biotechnology Association and the Association of Biotechnology Companies both small Washington-based biotechnology trade (and lobby) organizations…
1990
September 14, 1990 – First gene therapy clinical trial was conducted on a 4-year-old girl named Ashanthi DeSilva to fix a genetic defect – TIMELINE, FDA: Human Gene Therapy – ARCHIVE
1975
February 24, 1975 – Asilomar Conference on Recombinant DNA is held – TIMELINE
- In 1975 Berg organised the Asilomar Conference on Recombinant DNA ( rDNA), to put safeguards in place as making hazardous microorganisms now became possible, the guidelines were adopted but have “have gradually been diluted and disregarded.”
- “Genetic engineering has always worried the general public. When scientists first learned to clone genes in the mid-1970s, public reaction ranged from antipathy to hostility. Opponents, fearing that genetically engineered bacteria might escape from a laboratory, shut down the research at Harvard University and the Massachusetts Institute of Technology for months. Twenty-five years ago, in response to public concern, American scientists organized a voluntary moratorium on certain types of gene engineering experiments until safety questions could be resolved.” FDA Human Gene Therapy- REF, Read more – HERE
1971
1971 – Gene Splicing Technique is Discovered – genetic engineering is born – TIMELINE,
- In 1975 US biochemist Paul Berg founded the gene-splicing technique which opened the door to the invention of recombinant-DNA technology i.e. genetic engineering
- The terms “recombinant DNA technology,” “DNA cloning,” “molecular cloning,”or “gene cloning” all refer to the same process (using plasmids) – READ