I’ll use this page to collect information about the Pfizer-BioNTech COVID-19 mRNA vaccine.
Who is Pfizer?
Pfizer is a muilti-national bio-pharmaceutical company with a long history of criminality, where no one goes to jail, they just pay out billions of dollars in fines. These costs are factored into sales and marketing plans! During the pandemic Pfizer’s CEO and spokesperson is Albert Bourla
Who is BioNTech?
BioNTech is a German biotechnology company who became a publicly listed company in 2019 and had never before brought a product to market (same with Moderna!). They held the technology patents for the mRNA vaccine platform, and needed to partner with a company for clinical trial work and manufacture – they chose to collaborate with Pfizer in March 2020 (except for China due to FoSun Pharma alignment).
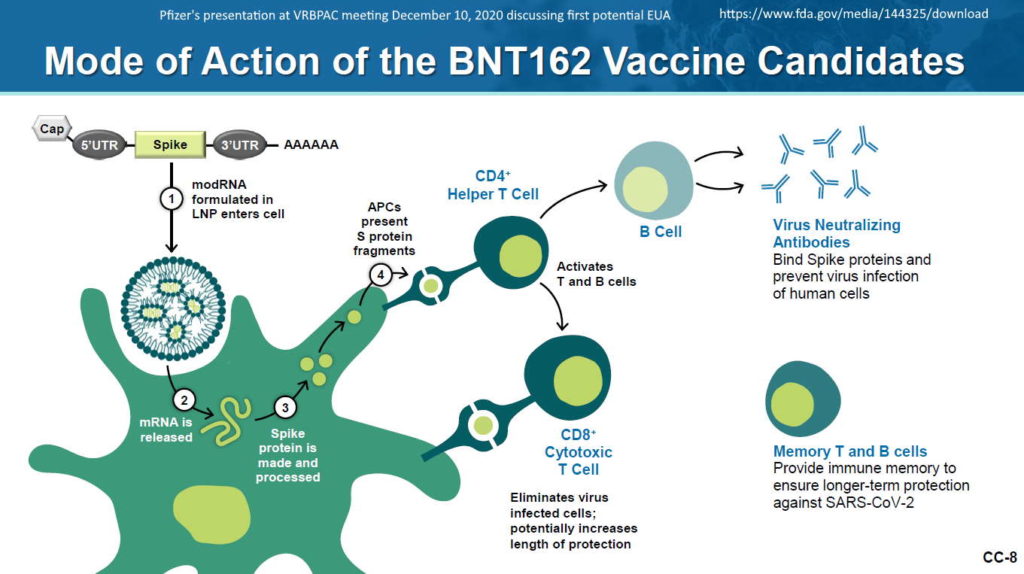
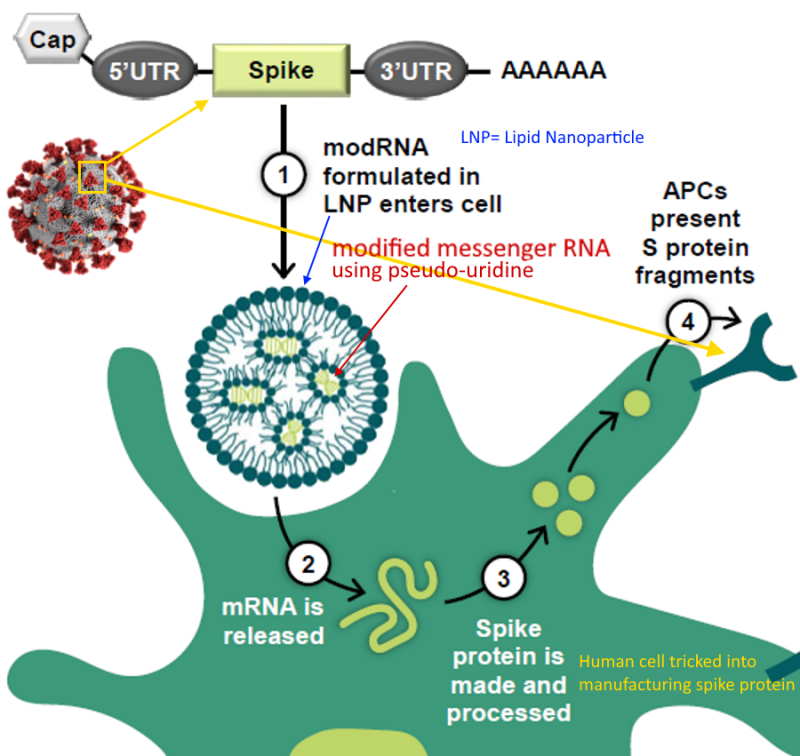
mRNA encased in LNP
…and called a “vaccine” because it elicits an “immune response” i.e antibody production
What is mRNA – Pfizer describes – HERE
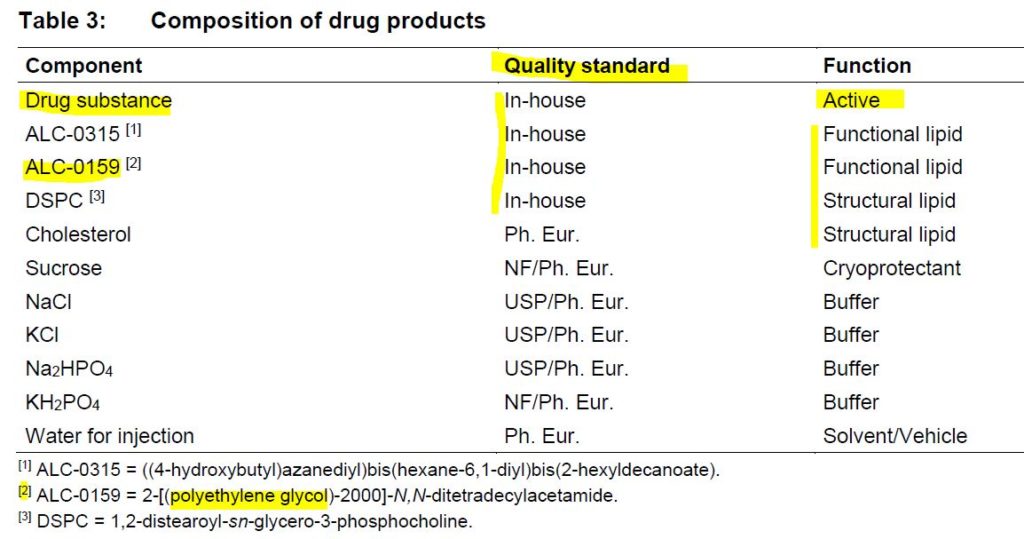
Pfizer-BioNTech uses Canada‘s Acuitas Therapeutics‘ lipid nanoparticle (LNP) delivery system called ALC-0315 and ALC-0159. – Press Release Nov 9, 2020 – HERE
The “Vaccine” Platorm
In an 87 page FOI document from BioNTech titled “INVESTIGATOR’S BROCHURE – BNT162” and dated August 12, 2020 (initially release dated March 25, 2020) it is clear that the company has 3 different RNA platforms under development, of which all are enveloped in a lipid nanoparticle (LNP).
The RNA formats or platforms refered to as “drug substances” are:
- nonmodified uridine containing mRNA (uRNA),
- nucleoside modified mRNA (modRNA), and
- self-amplifying mRNA (saRNA)
In their summary statement BioNTech state:
“The development of a ribonucleic acid (RNA)-based vaccine encoding a viral antigen that is translated by the vaccinated organism to protein to induce a protective immune response provides significant advantages over more conventional vaccine approaches.” Where BioNTech is exploring the 3 different platforms.
BNT162 is the base-name the company gives to any “RNA drug product” encapsulated in the same LNP.
The drug product candidate to go through the “vaccine” trials and get emergency use athorisation was BNT162b2, a modRNA product.
As of Aug 2020 – The primary pharmacology of the BNT162 vaccine candidates was evaluated in a range of non-clinical pharmacology studies in vitro [petri dish] “to confirm functionality of the RNA” and see if the antigen would be expressed and in vivo“[animal studies] – “to benchmark the different vaccine antigens and to provide proof-of-concept, i.e., to demonstrate that BNT162 vaccines can induce an anti-SARS-CoV-2 immune response” done in mice.
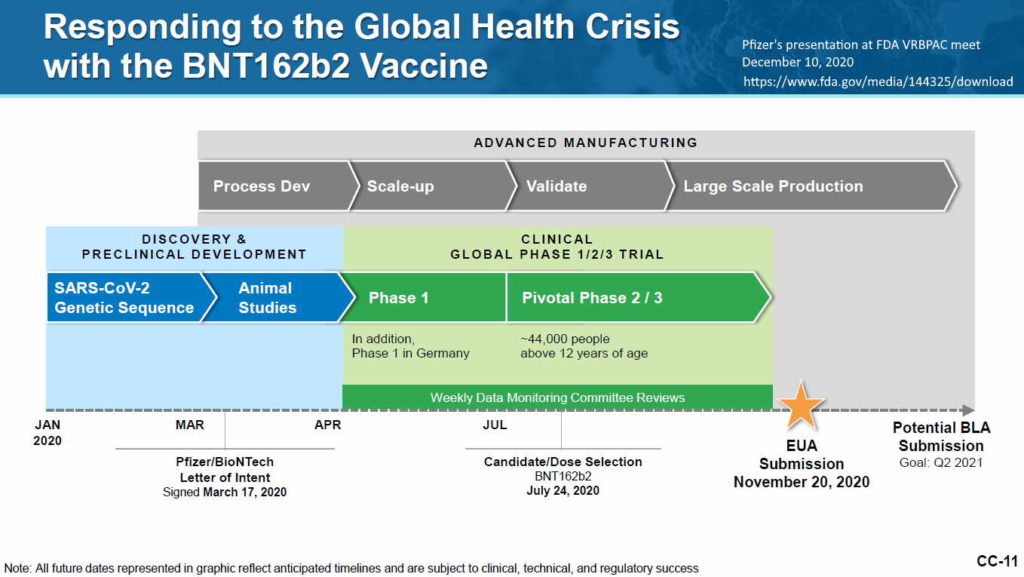
The Experiment
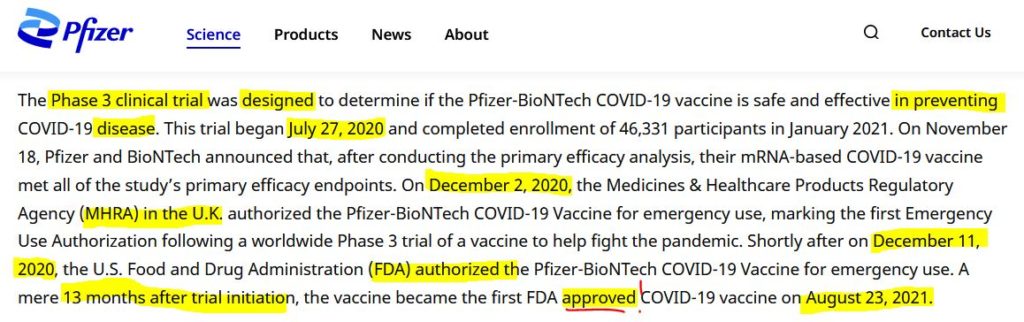
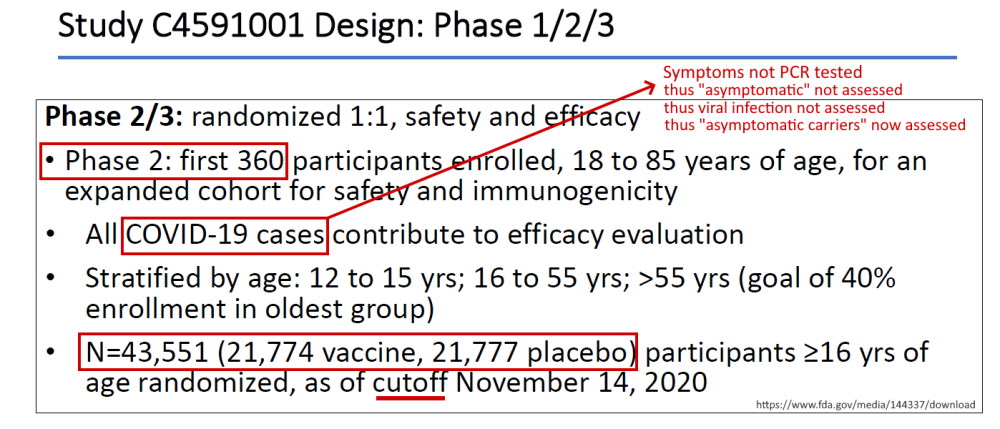
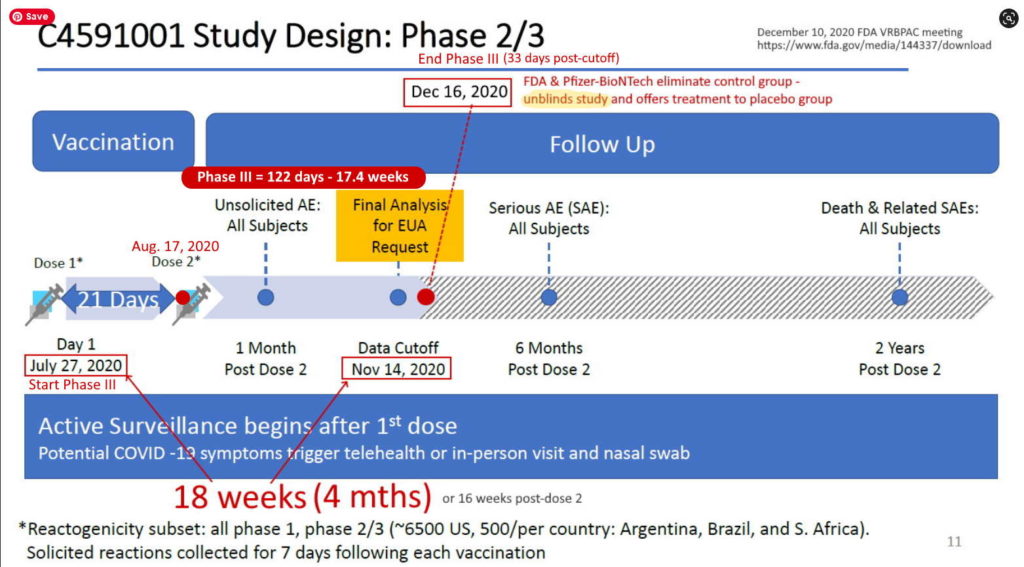
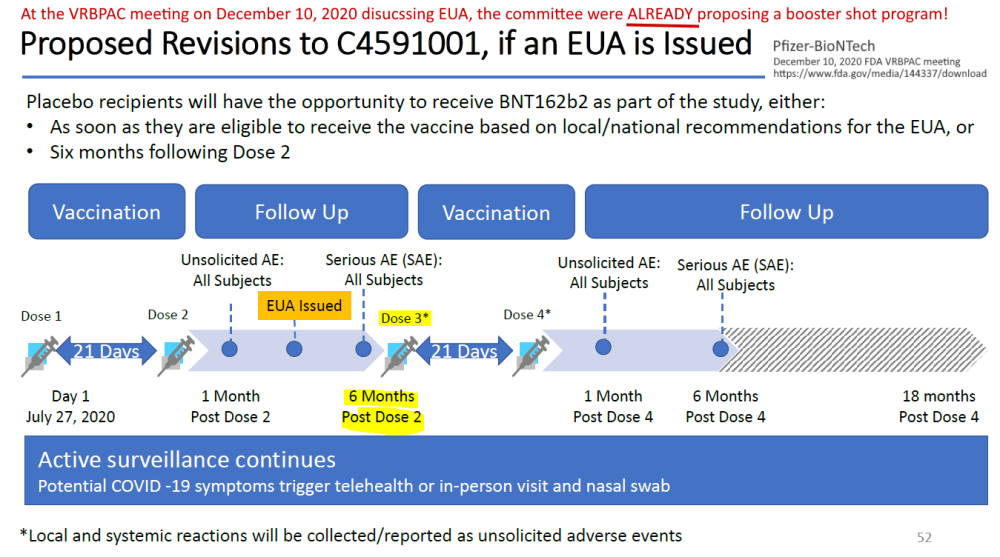
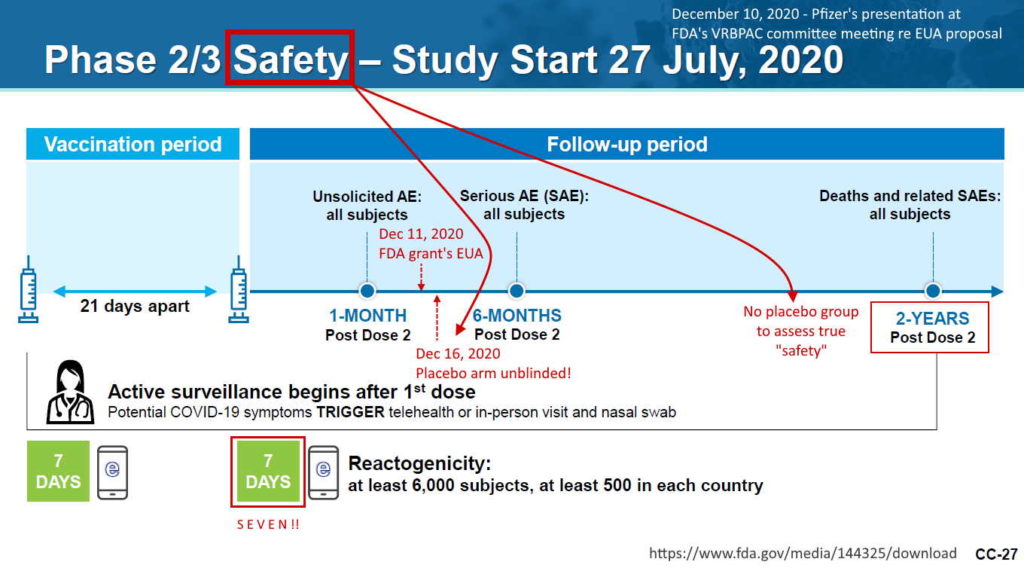
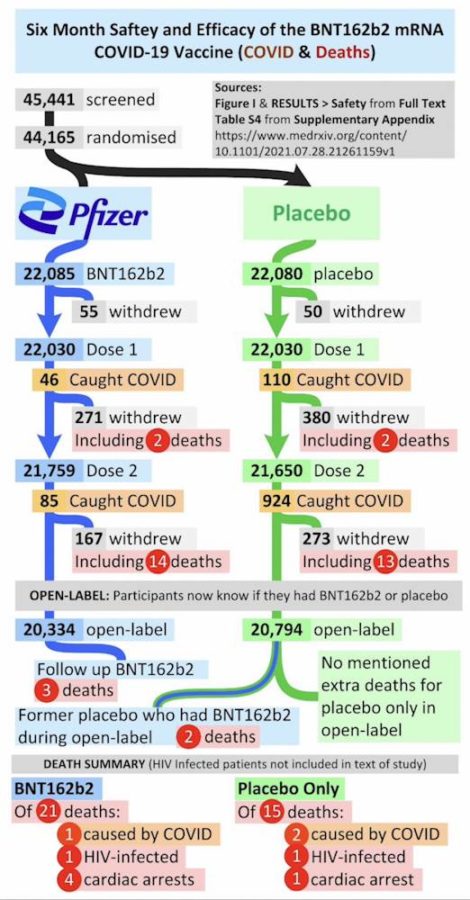
Pfizer’s phase 3 double blind placebo controlled clinical trial started on July 27, 2020 and was unblinded (ended) on December 16, 2020 – with barely 5 months of “long term safety data”, though there are whistleblower claims that Pfizer unblinded during the trial phase. The control group was eliminated, so all we have now to find out about long-term safety is trends in post-marketing surveillance data. The experiment is on going.
COMIRNATY and EUA product are “legally distinct”
The Pfizer-BioNTech COVID-19 Vaccine (Emergency Use Authorised) and COMIRNATY (COVID-19 Vaccine, mRNA) “are legally distinct with certain differences that do not impact safety or effectiveness”, state the FDA.- READ COMIRNATY has not been manufactures or supplied in the US, only the EUA labelled product has been. The latter has liability protection, the other doesn’t!

Origins of the name Comirnaty
An oncomir is a microRNA (miRNA) that is associated with cancer. In the name ‘Comirnaty’, miRNA itself is bookended between the ‘co’ (for “ComiR” or “miRNA”) and ‘ty’. – CREDIT
Pfizer’s Clinical Trial data publications:
- April 30, 2020 – ClinicalTrials.gov identifier: NCT04368728 [Phase 1/2/3] Safety, Tolerability, Immunogenicity, and Efficacy of RNA Vaccine Candidates – READ, ARCHIVE, Protocol C4591001 – PDF
- 2 month report, Dec 10, 2020 (orig.) – ARCHIVE, updated vers. Dec 31, ’21 – READ
- 6 month report, Sept 15, 2021 (orig.) – ARCHIVE, updated vers.Nov 5,’21 – READ
COMIRNATY- covid-19 vaccine, mrna injection, suspension Pfizer Laboratories Div Pfizer Inc – LABEL, ARCHIVES
Comirnaty – WEBSITE
Pfizer COVID-19 Press Releases – HERE
A ‘vaccine’ that ‘immunizes’ the person against symptoms not the pathogen!
Articles and video’s in reverse chronological order
Content is continuously being added, there is a backlog!
2024
July 10, 2024 – Times of India: Pfizer’s key scientist Mikael Dolsten, who developed Covid vaccine, to step down – after a more than 15-year career at the company – READ, Economic Times – READ
- Dolsten joined the company through its $68 billion acquisition of Wyeth in 2009, and became chief scientist 2010
May 31, 2024 – OpenVAET, et al : Pfizer/BioNTech C4591001 Trial – Audit Report – v1 (2024-05-31) – Reanalysis of the data and anomalies inventoried – READ This report is the synthesis of the work, and the product of a collaborative effort between multiple authors.
- “This review aims to address significant anomalies and discrepancies that have surfaced from the examination of the data from the Pfizer/BioNTech C4591001 trial, which could have profound implications for public trust and regulatory standards, should they not be adequately and transparently investigated.”
- Brook Jackson Whistleblower testimony of “systemic issues including lack of proper informed consent, enrollment of ineligible participants, data fabrication, and regulatory non-compliance”
- The case of Dr. Augusto Roux, a trial participant in Argentina
- Discrepancies in Randomization Figures from Analysis of two trial investigator files
- Missing trial subject identifiers suggestin non-random data handling issues, which could indicate deliberate subjects data deletion
- Delayed reporting of deaths in the Emergency Use Authorization (EUA) figures.
- Anomalies relating to Protocol deviations contrary to standard clinical trial practices, showed significant disproportions in treatment groups.
- Instances of unreported, under-reported and misclassification of adverse events were identified – highlighting violations of Good Clinical Practices (GCPs)
- Testimony revealing “systemic exploitation of protocol weaknesses, leading to widespread re-qualification of adverse effects as COVID-19 symptoms.
- Two distinct manufacturing processes (Process 1 used for clincal trial and Process 2 used for commercial supply and mass distribution). Process 2 was tested on only 252 recipients. The planned comparative study between the two processes aimed to assess safety and immunogenicity in participants aged 16-55 but was ultimately not conducted.
March 15, 2024 – Pharma Files Substack: Pfizer’s “Project Vax” Event in Singapore – Staffer Admits to Avoiding mRNA Jab during a Pfizer Vaccination campaign – (leaked audio) Pfizer Event Rep : “Honestly, I took Sinovac! hehehe” – READ, WATCH
- Pfizer propaganda poster and make a “Pledge” to get vaccinated! – TWEET
January 30, 2024 – Ed Dowd/ Chief Nerd on X: Pfizer stock continues to tank allegedly due to COVID constriction! Wall Street Analyst urging folks to look at Pfizer as a buy due to cancer growth potential – WATCH, Turbo Cancer – READ
January 12, 2023 – Euractiv: Pfizer officials could be excluded from the European Parliament says European Parliament’s special committee on COVID (COVI committee) – following company’s lack of transparency in vaccine purchase contracts during the pandemic – READ
January 11, 2024 – Vigilant News: Pfizer Makes Big Bet that latest COVID variant could trigger a “Heart Failure Pandemic” – READ
- Pfizer recently acquired Arena Pharmaceuticals, a firm specialising in developing treatments, particularly for heart inflammation conditions like myocarditis and pericarditis!
January 9, 2024 – Epoch Times | Facts Matter: 8th Shot of mRNA Vaccine, and the Coming ‘Heart Failure Pandemic’ – WATCH
- Re article in The American Association of Retired Persons (AARP) magazine
Latest dominant variant of SARS-CoV-2 is called JN.1, an offsping of earlier Omicron variant – where symptoms mimic the seasonal flu
2023
December 4, 2023 – ICAN: Final Batch of Pfizer Documents for Ages 16+ (According to FDA) Finally Released to the Public – READ,
October 31, 2023 – Global News: Pfizer reports 1st quarterly loss since 2019 on weak COVID product demand – READ, Dr Wolf – WATCH
- Pfizer urges investors to focus on non-COVID products such as new RSV vaccine [which has been on the increase since the rollout of their mRNA COVID-19 vaccine!]
October 20, 2023 – Sasha Latypova Substack: Breaking: Pfizer is going under the bus…Health Canada miraculously “found” SV40 promoter in Pfizer vials! So many governments are suddenly finding things that have been lost for years… – READ
- Pfizer stocks falling, Pfizer staff layoffs
October 13, 2023 – CNBC: Pfizer slashes full-year earnings and revenue guidance as Covid treatment, vaccine sales slump – READ, READ, AP – READ
October 12, 2022 – Euractiv: European Parliament’s special committee on COVID (COVI committee): Pfizer remained vague about the opacity of its vaccine purchase contracts and the text messages exchanged with European Commission President Ursula von der Leyen – READ
October 10, 2023 – Epoch Times: EXCLUSIVE: Health Canada Confirms Undisclosed Presence of DNA Sequence in Pfizer Shot –READ, TWEET, CREDIT
- Simian Virus 40 (SV40) DNA sequence in Pfizer COVID-19 vaccine, which the manufacture had not previously disclosed
August 25, 2023 – Epoch Times: EXCLUSIVE: Health Canada Not Concerned About Scientists’ Finding of Plasmid DNA Contamination in COVID Shots – READ
- 2 scientists (Kevin McKernan and Dr. Phillip J. Buckhaults) independently raised red flags about ~18 to 70 times greater than acceptable limits of plasmid DNA contamination in mRNA shots, potentially harmful to human genome
August 8, 2023 – Reuters: BioNTech reduces drug development spend as COVID vaccine sales plunge – READ, CREDIT
August 3, 2023 – Australian Senate Public Hearings: COVID-19 Vaccination Status (Prevention of Discrimination) Bill 2022 and the Fair Work Amendment (Prohibition COVID-19 Vaccine Discrimination) Bill 2023 – WATCH, TWEET, Sen Hanson – EXCERPT, Sen Antic (Q 4 Moderna)- EXCERPT
Australian Senators vs Pfizer – Video excerpts – HERE , vs TGA – HERE(credit Penny Butler)
- Australian Senate will question Pfizer and Moderna about why vaccines were mandated when there was no evidence they stopped transmission.
- Pfizer Australia Representatives
- Dr Krishan Thiru, Country Medical Director, Pfizer Australia
- Dr Brian Hewitt, Head of Regulatory Sciences, Pfizer Australia
- Australian Senators vs Pfizer – Video excerpts and more – HERE
August 1, 2023 – Fierce Pharma: As COVID revenues disappoint, once-high-flying Pfizer looks at possible cost cuts – READ, CREDIT
July 28, 2023 – Daily Clout: Pfizer’s Comirnaty mRNA COVID Vaccine Manufacturing Has Dropped by 89% Due to Decrease in Demand – READ
- The Follow the Money Team’s new report on Pfizer’s financials, based on Pfizer’s Q1 2023 reporting- PDF
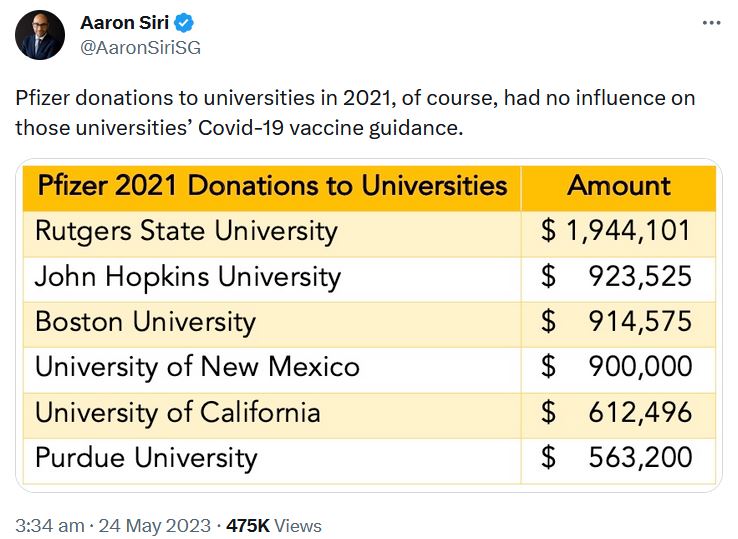
May 24, 2023 – Aaron Siri on Twitter: Regarding Pfizers 2021 Funding Report – US Medical, Scientific, Patient and Civic Organization Funding Report: FY 2021- PDF, THREAD
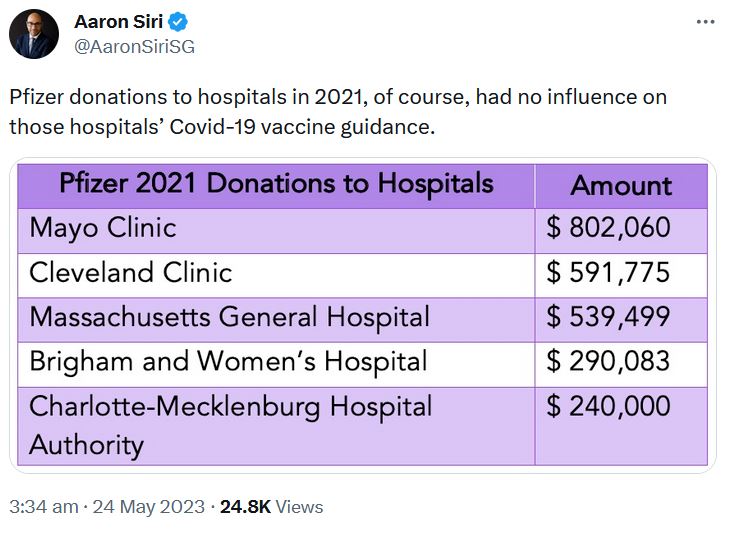
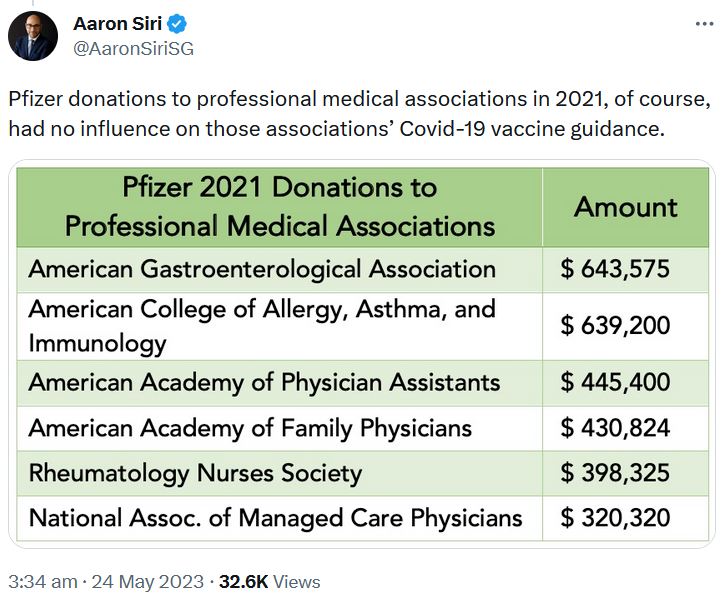
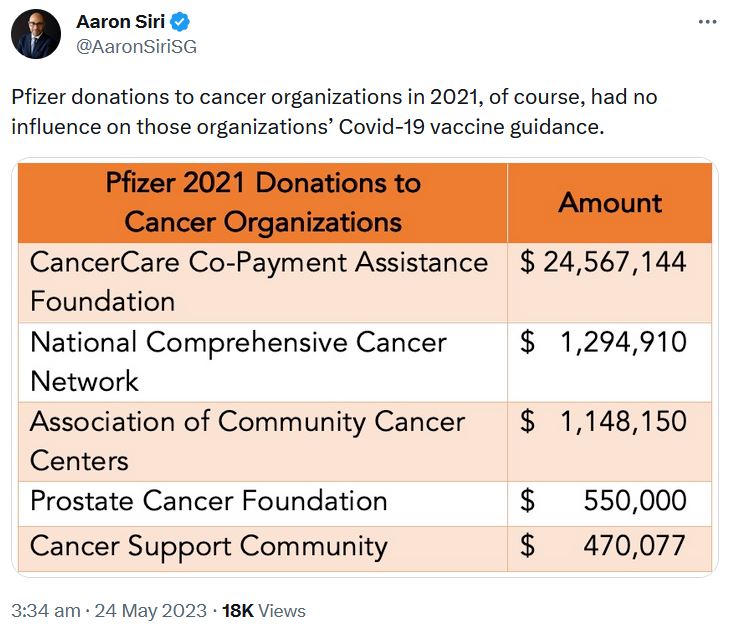
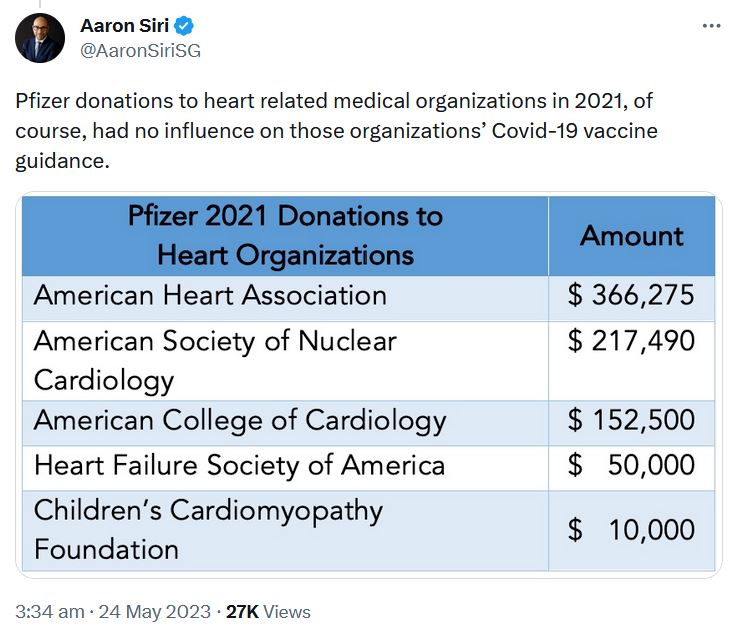
May 17, 2023 – UncoverDC: Good for Pfizer: Biden Nominates Bertagnolli for NIH Director – Between 2015-2021, Dr. Monica Bertagnoll received 89% of her Cancer grant funding from Pfizer, totalling $290.8 million – READ, Pfizer is not expanding into Cancer mRNA vaccine!
May 16, 2023 – Jackanapes Junction Substack: Reading The Pharma Racket Writing on The Wall – Healthcare clinic advertising public health annuncement sponsored by Pfizer warning of heart issues – READ, TWEET
- “Public Service,” it’s written. “Sponsored by and on behalf of Pfizer which is solely responsible for the content.”
- “Atrial fibrillation increases the risk of stroke by 5 times.”…”Ask the medical staff today about treatment for high blood pressure and atrial fibrillation?”[mRNA spike vaccine side effect but let’s not mention that]
- Pfizer has a new drug not yet available “that is supposed to directly treat atrial fibrillation” – TIKOSYN- dofetilide – PDF
- “So basically Pfizer creates the #1 selling pharmaceutical product in the world, the Comirnaty COVID vaccine, which apparently can cause atrial fibrillation and high blood pressure. It then turns around and pays for
advertisementspublic service announcements to increase sales of its products that are used to treat the problems caused by its vaccine. (Direct to consumer pharma ads are not allowed in Israel.)”
May 15, 2023 – Chief Nerd: Pfizer CEO Albert Bourla Says the Fallout From Vaccine Mandates Is Hurting Their Reputation Score – EXCERPT
May 12, 2023 – Dystopian Down Under | Rebekah Barnett: RED FLAG 🚩 Pfizer advocates for constitutional change in Australia – Voice to Parliament, brought to you by Pfizer – READ “All roads lead to ESG”
- Pfizer has weighed in on an upcoming referendum in which Australians will vote on whether to change their constitution.!
- May 11, 2023 – Pfizer (not an Australian citizen) pledged to support a “Yes” vote!!!! – PRESS RELEASE – PDF, WEB,

- Following excessive vaccine orders that were not used, “Now, it’s Pfizer’s turn to add value to the Australian Government’s agenda. As mentioned at the top, the sitting government is leading the YES campaign for the Voice referendum.”
May 11, 2023 – Pfizer Australia PRESS RELEASE: PFIZER PLEDGES SUPPORT FOR ULURU STATEMENT AND AN INDIGENOUS VOICE TO PARLIAMENT – PDF (Re referendum to Australia’s Constitution!), Website – READ, ARCHIVE
- “Pfizer began a journey of reflection three years ago [as the pandemic “opportunity” unfolded] which culminated in a commitment to reconciliation and the release of its Reflect Reconciliation Action Plan (RAP).”
- March 22, 2022 – PRESS: Pfizer launches its first Reconciliation Action Plan, commits to closing the gap – READ
April 13, 2023 – Anandamide Substack: Sequencing the Pfizer monovalent mRNA vaccines also reveals dual copy 72-bp SV40 Promoter – READ
April 10, 2023 – Meryl Nass Substack: FOIA’ed email from FDA …FDA improperly issued a COVID [Pfizer] vax license in order to impose mandates – READ
- The July 21, 2021 email is from Marion Gruber to Janet Woodcock (Acting Commissioner of FDA) and Peter Marks (Director of FDA’s vaccine center CBER) – She was explaining why issuing a license in August 2021 was not possible” But on August 23, 2021 they issued it anyway – TIMELINE
- The last week Aug 2021 Gruber quit/resigned/was fired? Allegedly over boosters – READ
“You expressed concern about the rising Covid cases in the US and globally, largely caused by the delta variant and stated your opinion that, absent a license, states cannot require mandatory vaccination and that people hesitant to get an EUA authorized vaccine would be more inclined to get immunized when the product is licensed.”
Marion Gruber email
April 8, 2023 – Red Pill News: Pfizer Whistleblower Debbie Bernal Revealed – WATCH – Pfizer was aware of myocarditis risk
- Debbie, with a background in healthcare consulting, applied for a job and landed one with Pfizer as “sales proccess expert and functional lead” for the virtual version of their sales
- She searched the companies internal portal and looked up “myocarditis” and was shocked to find internal reaseach studies and powerpoint presentations – it made her realise that internally Pfizer staff were perfectly aware of the myocarditis side effects from the COVID-19 jab, especially in young boys
March 20, 2023 – Cranmer’s Substack: Pfizer Vaccine Approval in New Zealand Under Scrutiny: A Retrospective Analysis – The government’s enthusiastic promotion of the Pfizer vaccine approval in New Zealand overstated the strength of the clinical assessment despite significant gaps in the data. – READ, CREDIT
March 15, 2023 – CHD | The Jerusalem Report w/ Ilana Rachel Daniel: Israel Pfizer Deal Revealed “A whistleblower shares the truth” – WATCH, FOIA’s submitted to Israel’s Ministry of Health – EXCERPT
- Flashback Dec 2022 – The Ministry of Health told the High Court: The agreement with Pfizer has disappeared – READ, then found additional 3 agreements
March 15, 2023 – Financial Times: Pfizer’s revised EU Covid vaccine contract meets resistance – Four member states including Poland say amended deal is not ‘fair solution’ to surplus of shots – READ, Redacted News – WATCH, BACKUP
- “@Pfizer has offered to change its #COVID19#vaccine contract with the EU [and] has agreed to extend its contract from 2023 to 2026 [but] it is insisting on payment for doses ordered in the contract that will never be manufactured, angering some governments.” – Mark Werner – TWEET
March 14, 2023 – FDA News Release: FDA Authorizes [EUA] Bivalent Pfizer-BioNTech COVID-19 Vaccine as Booster Dose for Certain Children 6 Months through 4 Years of Age – READ

March 14, 2023 – FDA Press Release: FDA Authorizes Bivalent Pfizer-BioNTech COVID-19 Vaccine as Booster Dose for Certain Children 6 Months through 4 Years of Age – READ
March 11, 2022 – Pfizer Press Release: Pfizer Completes Acquisition of Arena Pharmaceuticals – READ, CREDIT
- “Pfizer just purchased Arena Pharmaceuticals, a company known for making drugs to treat heart conditions like myocarditis and pericarditis. Arena is also developing two new cardiovascular assets: temanogrel for microvascular obstruction; and APD418 for acute heart failure.” – @BenSwann_ – TWEET
- March 28, 2023 – UPI News: Heart attack risk may skyrocket by 8 times for those with hidden ailment – READ, The Last American Vagabond – WATCH
March 10, 2023 – OpenVAET with Josh Guetzkow: Pfizer/BioNTech C4591001 Trial – How many mathematical proofs of fraud in this trial do we need, for independent inquiries to finally start …? – READ
March 6, 2023 – Daily Clout: Report 59: The Flawed Trial of Pfizer’s COVID-19 mRNA “Vaccine.” 90% of Original Placebo Group Received at Least One mRNA Injection by March 2021 – Linnea Wahl – READ
March 6, 2023 – ICAN: ICAN’s Attorneys Uncover Early Pfizer Vaccine Study Revealing Alarming Systemic Reactions in Rats – re June 2020 report – READ
- “While Pfizer claims in the report that the rats tolerated the vaccines “without evidence of systemic toxicity,” its detailed findings indicate that was anything but the truth,…” all issues “show effects beyond the injection site”.
- “Of particular concern is the increased fibrinogen concentration; fibrinogen is made in your liver and helps your blood clot. Increased fibrinogen is associated with blood clotting, heart disease, blood vessel dysfunction, and stroke. These issues were also seen with the dose level that was eventually licensed.
- In the short 6 week study, two rats died “during the study (about 1% of those injected with vaccine), but, predictably, the Pfizer paid researchers, despite being unable to determine a clear cause of death, simply assumed that the deaths were caused by stress from blood draws and therefore were not caused by the vaccines (despite the fact that one of the dead rats had an enlarged spleen, enlarged adrenal glands, and an enlarged iliac lymph node)!”
February 23, 2023 – Josh Guetzkow Substack | Jackanapes Junction: The Pfizer Clinical Trial in Argentina Was a Military Operation – And Augusto Roux has the contracts to prove it – READ
February 21, 2023 – Conservative Review: Horowitz: Major German paper reveals Pfizer fabricated clinical trials to cover up deaths – READ, German news Die Welt – ARCHIVE, (use google translate)
- “All those sudden deaths, heart attacks, and strokes we’ve been witnessing over the past two years were indeed observed during the Pfizer clinical trial…The company simply covered up the severe adverse events by kicking those participants out of the trial and/or suggesting without evidence that the deaths had nothing to do with the experiment.
- Clinical Trial: ““Patient no. 11621327” … found dead from a stroke in his apartment just three days after the second dose. Typically, with a novel product in trial, any death – even one not so sudden – makes the product suspect until it is proven innocent. Yet in this case, Pfizer simply dismissed the death as not related to the vaccine, just as the company did with Patient #11521497, who died 20 days later from cardiac arrest.”
- Patient Number 12312982 is 36 year old attorney Augusto Roux, one of 6,000 Buenos Aires Phase 3 trial participants, he “was severely injured with pericarditis and liver damage. Instead of being recorded as a severe adverse event, he was marked as having had COVID (even though he tested negative [several times]) and was summarily removed from the trial.” Alemán Hospital discharge report stated”Adverse reaction to the coronavirus vaccine (high probability)” – To the FDA Roux was registered as an unvaccinated, COVID-19 case and never registered as a pericarditis case!!!!
- “Overall, 21 participants in Pfizer’s phase 3 trial died, as compared to 17 in the control group before they were unblinded, which should have been a red flag before the shot ever took off.”…
February 14, 2023 – Alison Bevege Substack | Letters from Australia: PACKED TO THE RAFTERS: Drs Peter McCullough, Pierre Kory & Melissa McCann stunned Sydney with a full-spectrum mRNA-disaster truth nuke – READ
- Dr Melissa McCann from Queensland revealed Pfizer got to proof-read a draft media release from Australia’s drug regulator the Therapeutic Goods Administration (TGA) before it was released to the public – [Wait What?] PDF
Febraury 5, 2023 – Demonic Grammy Awards 2023 “brought to you by Pfizer” – WATCH
February 1, 2023 – CNN: The Covid sales boom is over for Pfizer – READ
- In 2022 Pfizer generated nearly $57 billion in combined sales from its COVID-19 vaccine and Paxlovid antiviral drug , equating to ~60% of the company’s total revenue …”But the boom appears to be over”.
January 31, 2023 – Daily Business: Pfizer loses $43 billion in worst month since 2009 – READ, Zero Hedge “down 15%” – READ
January 31, 2023 – NY Post: Pfizer forecasts big drop in revenue after record $100M COVID-led haul – READ, but respiratory syncytial virus (RSV) and an mRNA flu vaccine are on the horizon!
January 29, 2023 – Geoff Pain Substack: Production of the Pfizer BioNTech mRNA jabs – Billions of Jabs, Trillions of Dollars projected. Let us look at what we know about the manufacturing of the Toxic Spike Protein coding packaged in the Toxic LNPs – READ
- Buying up manufacturing facilities in preparation?
- Manufacturing Process 1 compared to Process 2 – “PCR amplified DNA template was used in Process 1, replaced by Linearized Plasmid DNA for Process 2.”
- “An interesting patent uses the word “Endotoxin” 117 times, discusses the expensive Magnetic Bead Purification method of deadly poison removal.” Used for Process 1, but what about for Process 2?
- Endotoxins are associated with anaphylaxis
- The European Medical Agency (EMA) has limits on the amount of bacterial endotoxin contamination in products resulting from production. Pfizer-BioNTech has to test and report for each batch.
- “Pfizer jab Manufacturing Process for BNT162b uses modified DH10B Escherichia coli Bacteria cells to grow the DNA used to make their Covid19 Spike Protein encoding mRNA. Interestingly these Bacteria have been designed to “invade” Human Cervix and Breast Cells. Even more interesting is the fact that Chloroquine assists the invasion”!
January 28, 2023 – Becker News: BREAKING: Pfizer RESPONDS to Project Veritas’ ‘Bombshell’ Undercover Video Showing Exec’s Remarks on ‘Mutating’ Viruses – READ, CREDIT, Dr Malone Substack – READ
January 28, 2023 – Gateway Pundit: Pfizer Quietly Releases Statement in Response to Bombshell Project Veritas Video – READ
January 27, 2023 (8:00 pm) – Pfizer PRESS RELEASE: Pfizer Responds to Research Claims – READ
January 27, 2023 – Epoch Times Health: FDA Quietly Changes End Date for Study of Heart Inflammation After Pfizer COVID Vaccination – READ,
- The study, as per August 2021 licensure was to assess “post-vaccination subclinical myocarditis, or heart inflammation, following a third dose of the vaccine in people aged 16 to 30” was due to be completed on June 30, 2022, with Pfizer submitting a final report to the FDA by December 31, 2022. That deadline passed, the FDA quietly changed the end date to June 30, 2023.
- FDA extended the date, because Pfizer asked for an extension! – READ
January 26, 2023 – Brian O’Shea Substack: Who is “Jordon Trishton Walker“? – Project Veritas recently released a video featuring “Jordon Trishton Walker,” Pfizer executive who revealed shocking new info. But finding anything about him is tough. Here is what I’ve found so far. – READ, THREAD, [May 2020 article by Jordaon Walker – ARCHIVE]
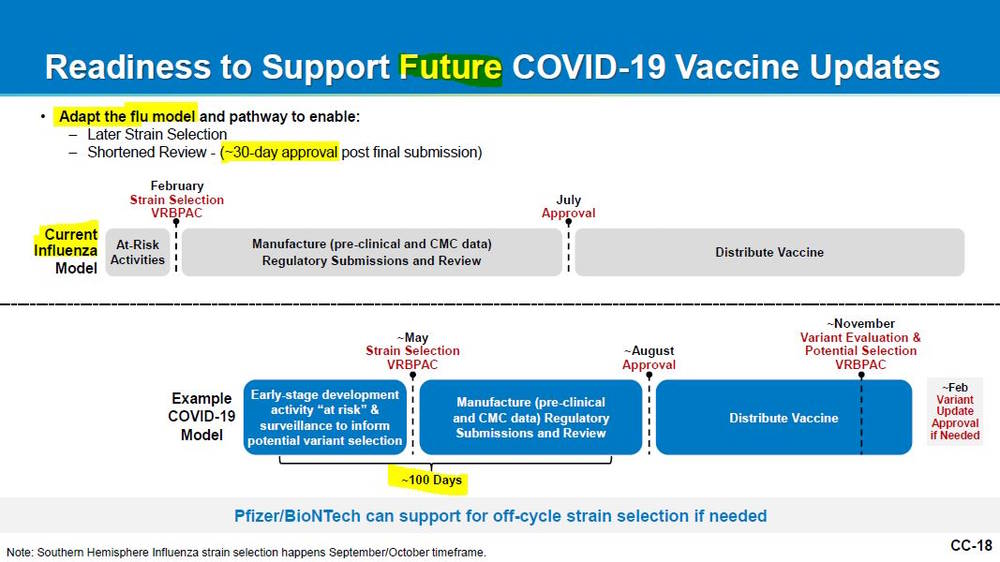
January 26, 2023 – FDA | VRBPAC meeting to Discuss Future Vaccination Regimens Addressing COVID-19 – PFIZER: Variant-modified vaccine experience to date -Readiness to support future COVID-19 vaccine updates – SLIDES, All meeting material – HERE, WATCH
January 25, 2023 – Project Veritas: Pfizer Executive [Jordon Trishton Walker]: ‘Mutate’ COVID via ‘Directed Evolution’ for Company to Continue Profiting Off of Vaccines … ‘COVID is Going to be a Cash Cow for Us’ … ‘That is Not What We Say to the Public’ … ‘People Won’t Like That’ … ‘Don’t Tell Anyone’ – WATCH,
- Bannons War Room – WATCH, James O’Keefe Reveals Massive Expose Of Pfizer’s Secret Gain-Of-Function Research – EXCERPT
- January 26, 2023 – Robert Malone Substack: Project Veritas has broken Pfizer’s Gain-of-Function Research Program Wide Open – Pfizer’s research is dangerous, immoral and must be shut down now – READ
Jan 22, 2023 – Russell Brand: I CAN’T BELIEVE THIS | Big Pharma’s Untold Truth Revealed By David Sirota – WATCH, EXCERPT, Lever News: Pfizer Pays To Change The Story – ARTICLE
- Big Pharma biz model – Use govt R& D money to buy back its’ own stock to boost/enrich it’s shareholders!
- “The Pfizer sponsorships [1] [i.e. for Expanding Adult vaccine access] are just the latest examples of how, when the news coverage gets rough, big businesses can pay corporate media to change the narrative and run counter-programming — with the hope that policymakers and consumers will be sufficiently distracted from the stories that really matter.”
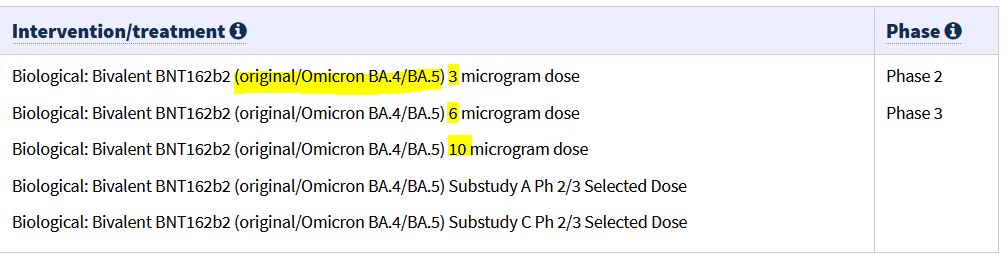
January 20, 2023 – TGA: Pfizer COVID-19 bivalent (COMIRNATY Original/Omicron BA.4-5 COVID-19 vaccine) booster dose vaccine – has been granted provisional approval for use as a booster dose in individuals aged 12 years and older – Pfizer’s bivalent COVID-19 vaccine: tozinameran and famtozinameran (COMIRNATY Original/Omicron BA.4-5 COVID-19 vaccine) is the 3rd bivalent registered in Australia – READ, ARCHIVE, All Provisional Registrations of CV19 vaccines – HERE, ARCHIVE
January 18, 2023 – Rebel News: Ezra Levant & Avi Yemini pummels Pfizer CEO, Albert Bourla, with questions [on His Unsafe and Ineffective mRNA Shots] at World Economic Forum in Davos and asks and he refuses to answer – WATCH, READ, BPR – READ, Gateway Pundit – READ
January 14, 2023 – NY Post: CDC investigating whether Pfizer COVID vaccine increases stroke risk for people over 65 – READ, Bloomberg – READ
January 14, 2023 – Geoff Pain Substack: Pfizer used Synthetic Life derived from US Bioweapons research for its mRNA trials – The US Bioweapons effort is known to have introduced the Furin Cleavage site into Coronavirus in 2005 – READ
January 13, 2023 – Brownstone Instutute: BioNTech (Not Pfizer) “Brazenly” Dodged Safety Testing of C19 Vax – they skipped the pharmacology studies – READ
- the preclinical phase of the drug’s development was “entirely under BioNTech’s control.”
January 13, 2023 – Zero Hedge: Deadline Passes For Pfizer To Submit Results Of Post-Vaccination Heart Inflammation Study To US Regulators – READ, ARCHIVE
- “Pfizer was required by the U.S. and Food and Drug Administration (FDA) to conduct multiple studies on its vaccine after the FDA approved the shot in August 2021 because regulators determined that without the studies, there would not be sufficient data to assess the “known serious risks of myocarditis and pericarditis,” or heart inflammation and a related condition….The FDA told Pfizer to carry out six studies, with various deadlines for completion and reporting final results to the agency. The first final deadline arrived on Dec. 31, 2022.”
January 6, 2023 – Fierce BioTech: Pfizer pivots from early-stage rare disease R&D, shifting to external innovation and putting assets up for sale – READ
- They will “pullback from new viral-based gene therapies and early-stage rare disease work in general”. [so much for being a gene-therapy leader announced in 2016 – REF], now wanting to be “best positioned to generate high-impact medicines and vaccines.”
January 4, 2023 – The Economist: BioNTech’s founder on the future of mRNA technology – Ugur Sahin tells us how mRNA will help fight pandemics and diseases such as cancer – READ
2022
December 25, 2022 – RT MAG: “We can’t locate a signed agreement with Pfizer”: Did the Netanyahu government and the Israeli MoH mislead the Israeli public and the world? New documents reveal – the deal between Israel and Pfizer was signed before their vaccine even received EUA – READ
December 23, 2022 – BioNTech PRESS RELEASE: BioNTech Initiates Phase 1 Clinical Trial for Malaria Vaccine Program BNT165 – READ, They “aim to develop the first mRNA-based vaccine for malaria prevention.” – ARTICLE
December 8, 2022 – PRESS RELEASE: Pfizer and BioNTech Receive U.S. FDA Emergency Use Authorization for Omicron BA.4/BA.5-Adapted Bivalent COVID-19 Vaccine in Children Under 5 Years – READ, Memorandum: approved without any clinical data – READ, Application submitted 4 days before on 5th Dec! – READ
December 1, 2022 – Vaccine: Surveillance of COVID-19 vaccine safety among elderly persons aged 65 years and older – Wong et al (FDA) – READ
- Four outcomes met the threshold for a statistical signal following BNT162b2 (Pfizer) vaccination including pulmonary embolism , acute myocardial infarction, disseminated intravascular coagulation, and immune thrombocytopenia. “No statistical signals were identified following vaccination with either the mRNA-1273 or Ad26 COV2.S vaccines.”
December 2022 [?] – Edge Radio Australia: Pfizer whistle-blower shows glowing evidence to Australian senator – Senator Malcolm Roberts and Melissa McAtee who worked at one of Pfizer’s manufacturing plants in the USA – WATCH
November 28, 2022 – Daily Sceptic: Pfizer CEO Found to Have Misled the Public Over Child Covid Vaccination by Pharmaceutical Watchdog – READ, The Telegraph ORIGINAL
November 26, 2022 -The Telegraph: Pfizer’s CEO rapped by regulator for making ‘misleading’ statements about children’s vaccines – Dr Bourla claimed youngsters aged five to 11 benefited from vaccination but the pharmaceutical watchdog said the remarks misled the public – READ
- Sept 2021 – “Dr Albert Bourla used an interview with the BBC last December to claim that “there is no doubt in my mind that the benefits, completely, are in favour of” vaccinating youngsters aged five to 11 against Covid-19.” – READ
November 22, 2022 – Karen Kingston Substack: FDA Documents Confirm Pfizer’s 2021 mRNA Vaccine Lots Had Different Formulations Based on Lot# – READ
November 18, 2022 – PRESS RELEASE: Pfizer and BioNTech Report New Data on Omicron BA.4/BA.5-Adapted Bivalent Booster Demonstrating Improved Immune Response Against Emerging Omicron Sublineages – READ
- Neutralizing antibody titers against emerging sublineages (BA.4.6, BA.2.75.2, BQ.1.1, and XBB.1 ) compared to original (Wuhan) vaccine – after 30 days
- Currently in US 30% cases = BA.5, but BQ.1.1 = 25% cases globally and increasing
November 16, 2022 – TGA: [Australia’s] TGA grants provisional determination to Pfizer’s COVID-19 bivalent (COMIRNATY BIVALENT OMICRON BA.4/BA.5) booster dose vaccine for 12 years and older – The new provisional determination means that Pfizer Australia Pty Ltd is now able to apply for provisional registration of this bivalent vaccine – READ [The formalities is that the TGA Secretary has to grant provisional determination before fast track PR can be moved into]
- Note On 27 October 2022, the TGA provisionally approvedthe bivalent vaccine with “Omicron BA.1 strains for use as a booster dose in adults”, this is for the BA.4/BA.5 strains.
November 14, 2022 – Daily Mail: Pfizer and Moderna launch trials to track whether health issues arise YEARS after getting their Covid vaccines – READ, but they got rid of the trial control groups in Dec 2020 – TIMELINE
November 10, 2022 – PRESS RELEASE: Pfizer and BioNTech Receive Positive CHMP Opinion for Omicron BA.4/BA.5-Adapted Bivalent COVID-19 Vaccine Booster for Children 5 Through 11 Years of Age in European Union – READ
- European Medicines Agency (EMA) Committee for Medicinal Products for Human Use (CHMP) has recommended bivalent COVID-19 vaccine for “marketing authorization” for children 5-11 years.
- The Omicron BA.4/BA.5-adapted bivalent COVID-19 vaccine is currently authorized in the European Union (EU) as a booster dose for individuals aged 12 years and older
November 9, 2022 – MHRA UK: Regulatory approval of Pfizer/BioNTech vaccine for COVID-19 – READ, ARCHIVE
November 9, 2022 – MHRA UK: Second Pfizer/BioNTech bivalent COVID-19 booster vaccine approved by UK medicines regulator – This adapted COVID-19 vaccine targets both the original virus and the Omicron BA.4 and BA.5 sub-variants. – READ
November 4, 2022 – PRESS RELEASE: Pfizer and BioNTech Announce Updated Clinical Data for Omicron BA.4/BA.5-Adapted Bivalent Booster Demonstrating Substantially Higher Immune Response in Adults Compared to the Original COVID-19 Vaccine – Phase 2/3 trial 30 days = “immune response” +55years – READ
October 28, 2022 – Biz News: Pfizer used Israel as “the world’s first laboratory” to study its Covid vaccine’s efficacy, NOT promote safety – READ,
- Jan 6, 2021 – “The Real World Epidemiological Evidence Collaboration Agreement” between Pfizer and Israel – ARCHIVE
October 22, 2022 – Quartz: Pfizer plans to sell its covid vaccine at a 10,000% markup in 2023 – Pfizer will charge as much as $130 per dose of Comirnaty, which costs an estimated $1.18 per dose to produce – READ. A 12,370% markup per dose – SUBSTACK, Daily Mail – READ, Reuters – READ
- The move comes as the nation moves away from government-controlled distribution of the jabs to rolling them out through the traditional US healthcare system.
- A transition in the distribution of the shots could come as early as early 2023
October 21, 2022 – RT Mag: Breaking: Leaked Video Reveals Serious Side-Effects Related to the Pfizer COVID-19 Vaccine Covered Up by the Israeli MOH – READ, SUBSTACK
- “The Israeli MOH had no adverse events reporting system for the entire year of 2021. They commissioned a research team to analyze the reports from a new system implemented on December 2021.
- A leaked video reveals that in June, the researchers presented serious findings to the MOH, that indicated long-term effects, including some not listed by Pfizer, and a causal relationship –
- so the Ministry published a manipulative report, and told the public that no new signal was found”
- “The researchers’ conclusions: The findings establish causality, and may lead to lawsuits”
October 20, 2022 – European Parliament: Ursula von der Leyen (EU President) is married to the German doctor Heiko von der Leyen… who is director of Orgenesis, which is owned by Pfizer… the same company that Ursula signed a 71 billion euro contract with to buy an astronomical 4.6 billion doses equating to 10 doses per EU citizen – TWEET
October 18, 2022 – VIDEO SUPERCUTS: Pfizer CEO Albert Bourla Repeatedly Lying About His Covid Vaccines – WATCH, Pfizer CEO saying that their vaccine protects “against transmission”, and then gradually changes his claims over time – GETTR, TWEET
October 12, 2022 – FDA authorizes bivalent COVID-19 vaccines for use as a booster dose in younger age groups. – READ, FDA official Pfizer vaccine page – ARCHIVE, LIVE
October 11, 2022 – MP Christian Terhes: Press conference after Pfizer CEO Albert Bourla refused to answer in front of European Parliament – WATCH
- Six members of the European Parliament held a press conference on October 11, 2022, one day after Albert Bourla, Pfizer CEO, refused to participate in the Covid committeee and answer questions.
October 10, 2022 – Rob Roos MEP Europe: In European Parliament COVID hearing, Pfizer representative J. Small admits the vaccine was never tested on preventing transmission before it entered the market – WATCH, TIMELINE
OAN – WATCH
September 29, 2022 – Politico: Pfizer CEO pulls out of testifying to EU Parliament COVID panel – “Pfizer Chief Executive Albert Bourla has pulled out of an appointment to testify before the European Parliament’s special committee on COVID-19, at which he was expected to face tough questions on how secretive vaccine deals were struck.” – READ
September 26, 2022 – PRESS RELEASE: Pfizer and BioNTech Submit Application to U.S. FDA for Emergency Use Authorization of Omicron BA.4/BA.5-Adapted Bivalent Vaccine Booster in Children 5 Through 11 Years of Age – READ, Clinical Trial “Phase 1/2/3 pediatric study” NCT05543616 (C4591048) – see below Sep 16!
September 24, 2022 – The Hill: Pfizer CEO tests positive for COVID-19 again – the second time he has contracted the virus in under two months, he is symptom-free and has not taken the new bivalent “booster” – READ
September 23, 2022 – FiercePhama: NFL season sees Pfizer kick off major Comirnaty COVID vaccine advertising offensive for teens, boosters -Upt til now Pfizer has been using a softly, softly approach when it comes to promoting its COVID-19 vaccine Comirnaty – READ
September 16, 2022 – CLINICAL TRIALS GOV: A Study to Learn About COVID-19 Bivalent BNT162b2 Omicron Containing Vaccine in Healthy Children (6 months – 12 years) – NCT05543616 [Phase 1/2/3 all in one!]- ARCHIVE, READ
- The companies (Pfizer/BioNTech) have also initiated a Phase 1/2/3 study NCT05543616 (C4591048) to evaluate the safety, tolerability, and immunogenicity of different doses and dosing regimens of the companies’ Omicron BA.4/BA.5-adapted bivalent COVID-19 vaccine in children 6 months through 11 years of age. -Sept 26 PRESS RELEASE
- Estimated Study Completion Date : February 18, 2025
September 12, 2022 – PRESS RELEASE: Pfizer and BioNTech Receive Positive CHMP Opinion for Omicron BA.4/BA.5-Adapted Bivalent COVID-19 Vaccine Booster in European Union – READ
September 1, 2022 – PRESS RELEASE: Pfizer and BioNTech Receive Positive CHMP Opinion for Omicron BA.1-Adapted Bivalent COVID-19 Vaccine Booster in European Union – READ
August 31, 2022 – PRESS RELEASE: Pfizer and BioNTech Granted FDA Emergency Use Authorization (EUA) of Omicron BA.4/BA.5-Adapted Bivalent COVID-19 Vaccine Booster for Ages 12 Years and Older – (Comprehensive list of information) – READ, – This is updated from June 25, 2022 press – ARCHIVE
August 31, 2022 – FDA PRESS RELEASE: FDA Authorizes Moderna, Pfizer-BioNTech Bivalent COVID-19 Vaccines for Use as a Booster Dose – READ
August 30, 2022 – The “novel” Lipid Nanoparticle compound ALC-0315 used in Pfizer jabs is so controversial that it has its own Wikipedia page. – GETTR, WIKI, The owner of the patent is Acuitas Therapeutics mentioned by Dr Dave Martin – HERE
August 29, 2022 – ICAN: ICAN Demands Answers About Death Discrepancies in Pfizer’s Clinical Trial – READ
August 26, 2022 – PRESS RELEASE: Pfizer and BioNTech Complete Submission to European Medicines Agency for Omicron BA.4/BA.5 Adapted Bivalent Vaccine – READ
August 23, 2022 – PRESS RELEASE: Pfizer and BioNTech Announce Updated COVID-19 Vaccine Data Supporting Efficacy in Children 6 Months through 4 Years of Age – READ
- “Sequencing of observed COVID-19 cases confirmed majority were caused by Omicron BA.2, broadening the evidence for efficacy across COVID-19 variants” – [yet the day before they submit EUA for bivalent in adults, and less than one month later, Sept. 16, BioNTech starts clinical trials with bivalent vaccine in childeren!]
August 23, 2022 – Business wire – Pfizer and BioNTech Submit Application to U.S. FDA for Emergency Use Authorization of Omicron BA.4/BA.5-Adapted Bivalent COVID-19 Vaccine – READ, ARTICLE
August 22, 2022 – Pfizer PRESS RELEASE: Pfizer and BioNTech Submit Application to U.S. FDA for Emergency Use Authorization of Omicron BA.4/BA.5-Adapted Bivalent COVID-19 Vaccine – READ, ARTICLE
August 20, 2022 – Rebel News: Bureaucrats responsible for public policy seemingly allowed to have private conflicts of interest – CANADA: Ontario’s key pandemic advisor, Chief Medical Officer of Health (CMOH) Dr. Kieran Moore, declared his own Pfizer conflict of interest. – READ
August 19, 2022 – Trial Site News: It Doesn’t Add Up! Pfizer’s mRNA Injections Dosage is Probabilistic. Conformity with Label Cannot Be Verified by Sasha Latypova – Same purple cap vial, labels one with 5 doses vs 6 doses yet same contents inside! – READ
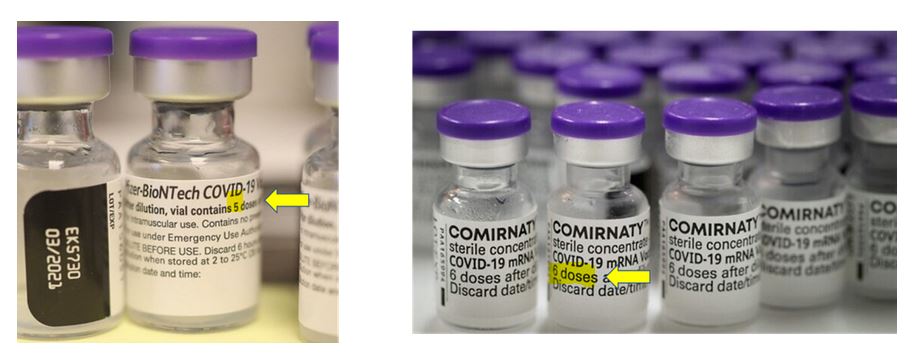
Australian TGA granted Provisional Registration to COMIRNARTY on January 25, 2021, a 6 dose vial (unusure of cap colour) – TIMELINE, Product Information – ARCHIVE
August 16, 2022 – Daily Clout: Pfizer, FDA, CDC Hid Proven Harms to Male Sperm Quality, Testes Function, from mRNA Vaccine Ingredients – READ
August 15, 2022 – PFIZER CEO TESTS POSITIVE for COVID-19! His vaccine failed to protect, so now he’s taking his drug Paxlovid! – Propaganda checklist – GETTR, NEWS, NEWS, NEWS
July 29, 2022 – Chief Nerd: Pfizer added some interesting language to one of their risks in their latest forward-looking guidance today, which was not there last quarter – GETTR
- “the impact of product recalls, withdrawals and other unusual items, including uncertainties related to regulator-directed risk evaluations and assessments, including our ongoing evaluation of our product portfolio for the potential presence or formation of NITROSAMINES” – REF: June 2, 2020 – FDA: What to Know and Do About Possible Nitrosamines in Your Medication – READ
July 29, 2022 – Geoff Pain on ReserchGate: Nitrosamines in Tromethamine – are analytical techniques adequate to enable real risk assessment? Tromethamine (Tris, THAM) is known to contain impurities than can be a potential source of Nitrosamine contamination.- READ, GETTR
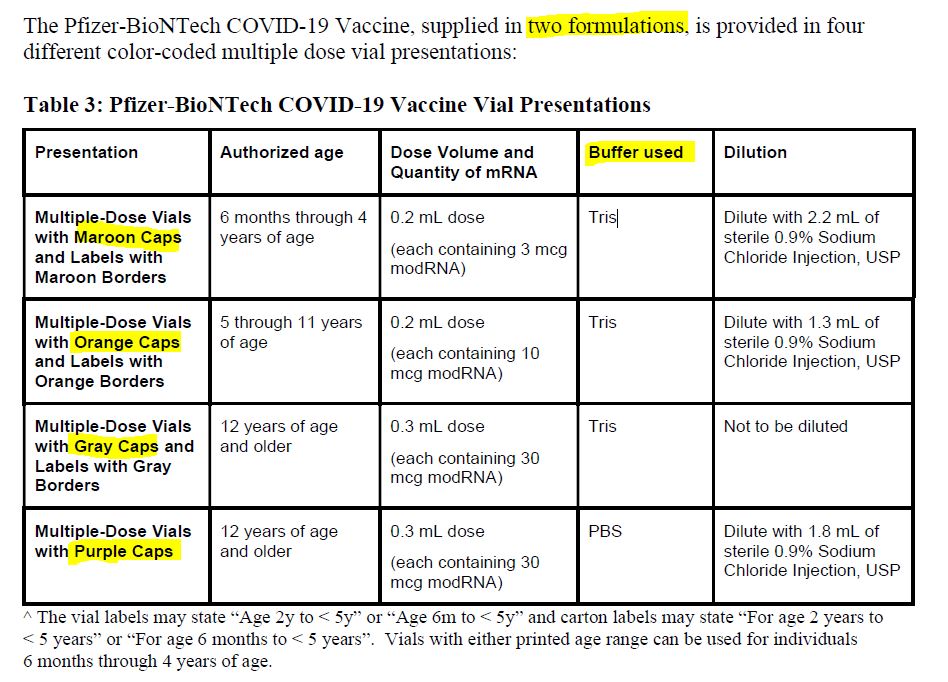
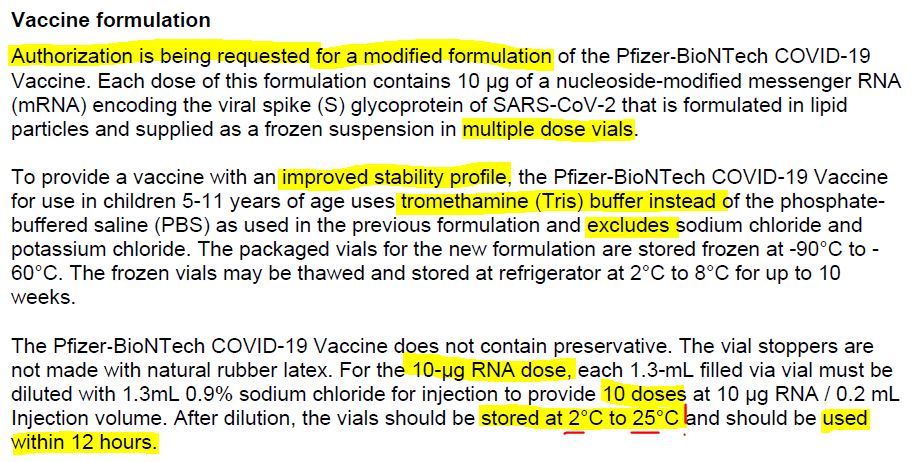
July 26, 2020 – Maryanne Demasi: FDA authorised new mRNA formula for kids without a clinical trial – READ
- “In October 2021, Pfizer requested the permission of the US Food and Drug Administration (FDA) to amend the formulation of its covid-19 vaccine for children aged 5-11 yrs” to now include “tromethamine (Tris) buffer” on December 17, 2021 the FDA granted the request.
- There were no clinical studies of the new formula in children. The FDA only looked at the “analytical comparability” and did not request any safety or efficacy studies of the newly formulated vaccine prior to it being rolled out to millions of children.
- 6mths -11 year old are only authorised to receive the vaccine with the new, untested, Tris buffer – SOURCE
July 23, 2022 – Health Canada’s response to a March 2022 letter expressing concerns over the shocking data revealed in the Pfizer documents…It appears Health Canada knew the information and just didn’t seem concerned enough to inform Canadians. – GETTR
July 27, 2022 – PRESS RELEASE: Pfizer and BioNTech Advance COVID-19 Vaccine Strategy With Study Start of Next-Generation Vaccine Candidate Based on Enhanced Spike Protein Design – “This next-generation bivalent COVID-19 vaccine candidate, BNT162b5, consists of RNAs encoding enhanced prefusion spike proteins for the SARS-CoV-2 ancestral strain (wild-type) and an Omicron variant.” – READ
July 26, 2022 – Conservative Review with Daniel Horowitz: The Man Who Holds the Key to Bringing Down the Pfizer Pfraud | Guest: Augusto Roux – Pfizer trial participant from Buenos Aires – LISTEN, 2023 article – READ
July 25, 2022 – PRESS RELEASE: Pfizer and BioNTech Announce Omicron-Adapted COVID-19 Vaccine Candidates Demonstrate High Immune Response Against Omicron – Candidates monovalant BA.1, bivalent BA.4 & BA.5 – READ
July 19, 2022 – PRESS RELEASE: Pfizer and BioNTech Complete Submission to European Medicines Agency for Omicron BA.1 Adapted Bivalent Vaccine Candidate – READ
July 13, 2022 – Daily Sceptic : Deaths After Vaccination in Pfizer Trial Not Fully Investigated, New Documents Reveal – READ
July 8, 2022 – CHD The Defender: Uruguay Halts COVID-19 Vaccine for Kids Under 13, Judge Demands Government Officials Turn Over Pfizer Contracts – READ
July 8, 2022 – PRESS RELEASE: Pfizer and BioNTech Announce U.S. FDA Approval of their COVID-19 Vaccine COMIRNATY® For Adolescents 12 through 15 Years of Age – COMIRNATY® is the first and only COVID-19 vaccine to be granted FDA approval for adolescents 12 years and older, following emergency use authorization in May 2021 – READ
June 30, 2022 – Trial Site News: Pharmaceutical Expert Says FDA Colluding with Pfizer and Moderna, Not Addressing Alarming Data with Alexandra Latypova (“Sasha”) – WATCH
June 29, 2022 – VOA News: Pfizer Signs New $3.2B Covid Vaccine Deal With US Government – READ
- “The average price per dose in the new deal is over $30, a more than 50% increase from the $19.50 per dose the U.S. government paid in its initial contract with Pfizer”
- Bide admin reallocated $10 BN funding to pay for vaccines
- 450 million doses distributed since first authorized in December 2020 of which >350 million of those doses have been administered.
June 29, 2022 – Business Wire: Pfizer and BioNTech Announce New Agreement with U.S. Government to Provide Additional Doses of COVID-19 Vaccine – US government buys 105 million doses of Pfizer’s mRNA vaccine for fall “booster” injections, with an option of 195 million more doses. “This may include adult Omicron-adapted COVID-19 vaccines” to be delivered “late summer 2022” – READ
June 29, 2022 – PRESS RELEASE: Pfizer and BioNTech Announce New Agreement with U.S. Government to Provide Additional Doses of COVID-19 Vaccine – “Omicron-adapted” vaccine – U.S. government will receive 105 million doses + 195 million additional – READ
June 28, 2022 – FDA advisory panel VRPBAC recommended that manufacturers update the design of their booster shots to include components tailored to combat the currently dominant omicron BA.4 and BA.5 variants. – HERE, SOURCE, TIMELINE
June 25, 2022 -PRESS RELEASE: Pfizer and BioNTech Announce Omicron-Adapted COVID-19 Vaccine Candidates Demonstrate High Immune Response Against Omicron – READ, SOURCE (referenced in authorisation press release – HERE)
- Monovalent vaccine from January 25, 2022 clinical trials
June 17, 2022 – Dr. Clare Craig Exposes How Pfizer Twisted Their Clinical Trial Data for Young Children (6mth – 4 years) – WATCH, READ
June 17, 2022 – PRESS RELEASE: Pfizer-BioNTech COVID-19 Vaccine Receives FDA Emergency Use Authorization for Children 6 Months through 4 Years of Age – READ
- Pfizer confesses to FDA that it really doesn’t know how the COVID-19 vaccine works – WATCH
June 11, 2022 – Team Enigma: Moderna and Pfizer: Reproductive Toxicology Studies from FOIA Documents – WATCH
June 4, 2022- The Dossier Substack: Ghost Shot: Pfizer quietly admits it will never manufacture original FDA approved COVID vaccines – Comirnaty is a legally distinct product from the emergency use authorization (EUA) shots, Comirnaty has never made its way to market. – READ
May 25, 2022 – World Economic Forum | Davos 2022: Conversation with Albert Bourla, CEO of Pfizer – WATCH
The first week we met in January of ’19 in California, and set up the goals for the next 5 years, and one of them was by 2023 we would reduce the number of people in the world that cannot afford our medicines by 50%, today this dream is coming reality.
Albert Bourla – [Poor English or double meaning?]
May 25, 2022 – Rounding the Earth Substack by Mathew Crawford: Pfizer Trial Fraud: The House of Cards Shakes – READ

May 23, 2022 – PRESS RELEASE: Pfizer-BioNTech COVID-19 Vaccine Demonstrates Strong Immune Response, High Efficacy and Favorable Safety in Children 6 Months to Under 5 Years of Age Following Third Dose – READ
May 22, 2022 – Rebel News | The Exra Levant Show: Pfizer CEO Albert Bourla tells WEF crowd about new microchipped pills: ‘Imagine the compliance’ – He’s spreading a Consiracy Theory about microchips! – WATCH
May 19, 2022 – American Thought Leaders: What did Pfizer know – What Are They Hiding?—Dr. Robert Malone on the Pfizer Documents and Evidence of Cardiotoxicity, Birth Defects, and the Rise in All-Cause Mortality – WATCH
May 18, 2022 – LifeSite News: Growing evidence suggests Pfizer committed widespread fraud in its COVID jab trials – READ, More – HERE
- May 9, 2022 – Steve Kirsch: Pfizer fraud or not? (Trial Sites 1231 and 4444) – Kirsch comments on the Jikkyleaks thread commenting on the Pfizer documents that appear to show clinical trial fraud. – WATCH
May 17, 2022 – PRESS RELEASE: Pfizer and BioNTech Granted U.S. Emergency Use Authorization for Booster Dose of Their COVID-19 Vaccine in Children 5 Through 11 Years of Age – READ
May 13, 2022 – Forbes: Pfizer’s Covid Vaccine Protection Against Omicron Fades Just Weeks After Second And Third Doses, Study Finds – READ
May 13, 2022 – JAMA: Neutralizing Antibodies Against the SARS-CoV-2 Omicron Variant (BA.1) 1 to 18 Weeks After the Second and Third Doses of the BNT162b2 mRNA Vaccine – Lassauniere et al – STUDY
May 7, 2022 – Jikkyleaks via Twitter – Another FOI request to the TGA is declined, but the refusal speaks volumes – re NSW Supreme Court case Kassam vs Hazzard vaccine mandate case, the TGA refused subpoena “for the animal studies relating to the Pflzer vaccines” – THREAD
- On the morning of the case, TGA’s lawyers didn’t appear, The sponsor’s lawyers did! “Mr Wong appears for Pflzer” – TWEET
May 3, 2022 – Maryanne Demasi: Is Pfizer’s FDA-approved COMIRNATY vaccine available in the US? – READ
May 2, 2022 – Judicial Watch: Pfizer/BioNTech Study Found Lipid Nanoparticles (LNP) Materials Outside Injection Site in Test Animals – Today received 466 pages from HHS – READ
- revealed: “a key component of the vaccines developed by Pfizer/BioNTech, lipid nanoparticles (LNPs), were found outside the injection site, mainly the liver, adrenal glands, spleen and ovaries of test animals, eight to 48 hours after injection.”
- Label a gene therapy product a “vaccine” and bypass regulatory hurdles justified because of WHO guidelines. “Safety Pharmacology” and “Pharmacodynamic Drug Interactions” can be deemed not necessary!
- “No safety pharmacology studies were conducted as they are not considered necessary according to the WHO guideline (WHO, 2005).”
April 26, 2022 – PRESS RELEASE: Pfizer and BioNTech Submit Application for U.S. Emergency Use Authorization for a COVID-19 Vaccine Booster Dose in Children 5 Through 11 Years of Age – READ
April 20, 2022 – Express UK: Vaccine study of 23 million shows risk of ‘heart problems’ from Moderna or Pfizer jab – READ
April 14, 2022 – PRESS RELEASE: Pfizer and BioNTech Announce Data Demonstrating High Immune Response Following a Booster Dose of their COVID-19 Vaccine in Children 5 Through 11 Years of Age – READ
April 8, 2022 – Kanekoa Substack: Pfizer’s Covid-19 Vaccine Documents Counter “Safe And Effective” Narrative – Nine-pages of side effects, 158,893 adverse events, 42,086 case reports, 1,223 fatalities in Pfizer’s first three months [of post marketing surveillance] and a record death rate among American millennials during the second half of 2021. – READ
April 6, 2022 – FDA’s Vaccines and Related Biological Products Advisory Committee (VRBPAC) – Dr. Peter Doshi‘s testimony on “Data Integrity and Regulatory Oversight” and the Pfizer Whistleblower – WATCH, SUBSTACK, The Expose – ARTICLE, BMJ investigation – HERE
March 29, 2022 – PRESS RELEASE: Pfizer and BioNTech Receive Expanded U.S. Emergency Use Authorization for an Additional COVID-19 Vaccine Booster in Individuals Aged 50 Years and Older – READ
March 27, 2022: Geoff Pain on ResearchGate: Tromethamine in experimental mRNA COVID-19 vaccines predictably causes severe side effects – Why is it still approved for use? – Tromethamine is an Endocrine Disruptor and causes Anaphylaxis. – READ
March 22, 2022 – Bright Health: Newly Released Pfizer Documents Reveal COVID Jab Dangers – What really happened in the first 90 days of the jab rollout? The first tranche of documents were released March 1, 2022 – READ
March 15, 2022 – PRESS RELEASE: Pfizer and BioNTech Submit for U.S. Emergency Use Authorization of an Additional Booster Dose of their COVID-19 Vaccine for Older Adults – READ
March 11, 2022 – CNBC: Pfizer CEO Albert Bourla on need for fourth Covid vaccine dose, ‘panvaccine’ and more – WATCH, TRANSCRIPT
March 3, 2022 – The Highwire Ep 257 : PFIZER’S COVID VACCINE DATA DUMP BEGINS – FOIA: First 10,000 pages – WATCH, FULL
March 3, 2022 – Pathology – Research and Practice – Case Report: Autoimmune mucocutaneous blistering diseases after SARS-Cov-2 vaccination: A Case report of Pemphigus Vulgaris and a literature review by Calabria et al – READ, PDF, SOURCE
February 17, 2022 – Market Place: BioNTech to ship mobile vaccine factories to developing countries – “modular factories assembled from shipping containers that produce the mRNA vaccine the company makes with Pfizer” – READ, Dr Nass – CREDIT
February 11, 2022 – PRESS RELEASE: Pfizer and BioNTech Provide Update on Rolling Submission for Emergency Use Authorization of Their COVID-19 Vaccine in Children 6 Months Through 4 Years of Age – READ
February 8, 2022 – The Times of Israel: Pfizer says it raked in $36,800,000,000 in COVID-19 vaccine sales in 2021, “making it among the best-selling drugs in history, and expects to bring in $50 billion from the vaccine and the Paxlovid therapeutic in 2022.” – READ
February 8, 2022 – PRESS RELEASE: Pfizer Reports Fourth-Quarter and Full-Year 2021 Results – “Full-Year 2021 Revenues of $81.3 Billion, Reflecting 92% Operational Growth” – PDF
February 7, 2022 – National File: University That Funds Biden’s Think Tank And Hosts FactCheck.Org Has Contract With BioNTech, Gets Paid For Vaccine Sales And FDA Approvals – University of Pennsylvania – READ
January 25, 2022 – NBC News: Pfizer starts clinical trial for omicron-specific Covid vaccine – a modified Covid-19 vaccine with omicron variant – READ, Pfizer CEO – WATCH
- Pfizer and BioNTech hope to enroll up to 1,420 healthy adults ages 18 to 55 in their clinical trial to look for “immune response” i.e. the production of an antibody. Three groups: 1) fully vaccinated, 2) fully vaccinated and boosted, and 3) unvaccinated but no saline placebo control group, so how can they assess “safety”?
January 25, 2022 – PRESS RELEASE: Pfizer and BioNTech Initiate Study to Evaluate Omicron-Based COVID-19 Vaccine in Adults 18 to 55 Years of Age (monovalent vaccine) – READ
January 24, 2022 – PRESS RELEASE: Pfizer and BioNTech Publish Data from Two Laboratory Studies on COVID-19 Vaccine-induced Antibodies Ability to Neutralize SARS-CoV-2 Omicron Variant – READ
- “Data published in the peer-reviewed journal Science, includes readouts of sera data from 51 vaccinated individuals that received two or three doses of BNT162b2 as well as a study evaluating the neutralization potential of serum antibodies from a subset of vaccinated individuals against the live virus.”
- “Both data sets… demonstrating that serum antibodies induced by BNT162b2 neutralize the SARS-CoV-2 Omicron variant after immunization with three doses.”
January 21, 2022 – News.com.au: Israeli officials were ‘surprised and disappointed’ vaccines did not stop transmission – READ
January 18, 2022 – Science: Neutralization of SARS-CoV-2 Omicron by BNT162b2 mRNA vaccine–elicited human sera – (by BioNTech et al ) – READ, PDF
January 12, 2022 – Express – Pfizer’s 75-year sealed vaccine ‘safety data’ to be released after judge ruling – PFIZER’s full vaccine “safety data” will be released in just months, rather than the 75 years they would have taken, after a US judge’s ruling. – READ
January 12, 2022 – PRESS RELEASE: Positive Top-Line Results of Pfizer’s Phase 3 Study Exploring Coadministration of PREVNAR 20™ With Pfizer-BioNTech COVID-19 Vaccine in Older Adults Released -PREVNAR 20™ (Pneumococcal 20-valent Conjugate Vaccine) with a booster dose of the Pfizer-BioNTech COVID-19 Vaccine! – READ
January 11, 2022 – NBC News: Omicron vaccine to be ready in March, Pfizer CEO says – As Covid-19 cases continue to surge, Pfizer CEO Albert Bourla says its new vaccine would target omicron and other variants already circulating. – WATCH
January 10, 2022 – CNBC: Pfizer CEO Bourla: Omicron-specific Covid vaccine will be ready by March – WATCH, TRANSCRIPT, Epoch Times – SOURCE
- “…a driver of finding solutions to unmet medical needs, and we have a very strong belief that the mRNA is a very powerful technology.”
- “…three more deals that we have announced, very important one with the Beam. Beam is a, is a leader in the base gene editing technology“
- “I wouldn’t underestimate also the agreement that we did with Codex. Codex has a technology that you can produce DNA, not through biological means …but with chemical….reduce the time of producing a very essential part of the overall manufacturing process for RNA vaccines from almost a month to a couple of days.”
January 10, 2022 – PRESS RELEASE: Pfizer Enters into Agreement with Acuitas Therapeutics for Lipid Nanoparticle Delivery System for Use in mRNA Vaccines and Therapeutics – READ, Acuitas Press – PDF, TIMELINE, ARTICLE
“Our swift delivery of the world’s first mRNA-LNP-based vaccine made clear the promise of mRNA-LNP technology,”
Mikael Dolsten, M.D., Ph.D., Chief Scientific Officer and President, Worldwide Research, Development and Medical of Pfizer Inc
January 8, 2022 – Reuters: ‘Paramount importance’: Judge orders FDA to hasten release of Pfizer vaccine docs – READ
January 7, 2022 – Washington Examiner: Judge scraps 75-year FDA timeline to release Pfizer vaccine safety data, giving agency eight months – FOIA for documents relied upon to license Pfizer’s COVID-19 vaccine – READ
January 7, 2022 – Town Hall: Federal Judge Smacks Down FDA’s Request to Release Pfizer Safety Data Over 75 Years – READ
January 6, 2022 – A federal court judge in the Northern District of Texas ordered the expedited release of all Pfizer documents relied upon by the FDA to authorise their COVID-19 vaccine. – READ, WarRoom Posse docuement findings – HERE, TIMELINE
January 3, 2022 – Kanekoa Substack: The Powerful Pfizer Presentation That Got Dr. Robert Malone Kicked Off Twitter – Pfizer’s own vaccine trial data shows an INCREASED risk of illness and death for the vaccinated. – READ
January 3, 2022 – PRESS RELEASE: Pfizer and BioNTech Receive U.S. FDA Emergency Use Authorization of COVID-19 Vaccine Booster for Individuals 12 Years of Age and Older – First EUA in the US for a COVID-19 vaccine booster in adolescents 12 to 15 years of age – READ
2021
December 23, 2021 – PRESS RELEASE: Pfizer and BioNTech Submit Updated Longer-Term Follow-Up Data of COMIRNATY® in Adolescents 12 Through 15 Years of Age to EMA – READ
December 20, 2021 – PRESS RELEASE: Pfizer and BioNTech to Provide European Union More Than 200 Million Additional Doses of COMIRNATY® to Help Meet Continued Need for Vaccine Supply – READ
December 17, 2021 – PRESS RELEASE: Pfizer and BioNTech Provide Update on Ongoing Studies of COVID-19 Vaccine – Evaluation of vaccine in children including 6mth to 5 yrs – READ
December 17, 2021 – UK Scientist Reveals Bombshell Data Analysis: Tracks Batches Of Pfizer, Moderna and Janssen, Finds “..Some Batches Are 50 Times Worse Than Others” – READ
How bad is my batch – WEBSITE by Craig Paardekooper
December 17, 2021 – FDA approves Pfizer-BioNTech change of formulation, because Tris buffers are a “commonly used buffer in other FDA-approved vaccines.” – HERE
December 16, 2021 – Canadian Covid Care Alliance CCCA: The Pfizer Inoculations For COVID-19 do More Harm Than Good – WATCH, SOURCE, SLIDES, The video that got Dr Malone kicked off of Twitter – ARTICLE
More Harm Than Good: This video of the Pfizer 6 month data shows that Pfizer’s COVID-19 inoculations cause more illness than they prevent. Plus, an overview of the Pfizer trial flaws in both design and execution.
December 15, 2021 – Kanekoa Substack: Pfizer’s History of Fraud, Corruption, and Using Nigerian Children as ‘Human Guinea Pigs’ – How did Pfizer manage to rebrand itself as the savior of humanity? – READ
December 9, 2021 – PRESS RELEASE: Pfizer and BioNTech Receive U.S. FDA Emergency Use Authorization of COVID-19 Vaccine Booster for Individuals 16 Years and Older – READ
December 8, 2021 – PRESS RELEASE: Pfizer and BioNTech Provide Update on Omicron Variant – READ
- Preliminary laboratory studies demonstrate that three doses of the Pfizer-BioNTech COVID-19 Vaccine neutralize the Omicron variant (B.1.1.529 lineage) while two doses show significantly reduced neutralization titers
- As 80% of epitopes in the spike protein recognized by CD8+ T cells are not affected by the mutations in the Omicron variant, two doses may still induce protection against severe disease
- The companies continue to advance the development of a variant-specific vaccine for Omicron and expect to have it available by March in the event that an adaption is needed to further increase the level and duration of protection – with no change expected to the companies’ four billion dose capacity for 2022
December 8, 2021 – Telegraph: Three doses of Pfizer vaccine ‘effective’ against omicron Covid variant – Data from Pfizer-BioNTech indicates three doses is as good against the new mutation as two doses is against the original virus – READ
December 1, 2021 – National Pulse: CONFLICT: Reuters Chairman is Pfizer Investor and Board Member – READ, CONTEXT, Trusted News Initiative – TIMELINE
- The chairman and former Chief Executive Officer (CEO) of the Thomson Reuters Foundation– James C. Smith – is a top investor and board member for pharmaceuticals giant Pfizer.
- In the last year alone, Reuters has published more than 22,000 articles mentioning Pfizer. The company has only published 8,191 articles related to Moderna, and 18,000 related to Johnson & Johnson. Many of the articles about Johnson & Johnson were negative in sentiment, unlike their Pfizer reporting.
November 25, 2021 – European Medicines Agency: Comirnaty COVID-19 vaccine: EMA recommends approval for children aged 5 to 11 – READ
November 22, 2021 – PRESS RELEASE: Follow-Up Data From Phase 3 Trial of Pfizer-BioNTech COVID-19 Vaccine Support Safety and High Efficacy in Adolescents 12 Through 15 Years of Age – READ
November 18, 2021 Aaron Siri Substack: FDA Asks Federal Judge to Grant it Until the Year 2076 to Fully Release Pfizer’s COVID-19 Vaccine Data [55 years] – READ
“So, let’s get this straight. The federal government shields Pfizer from liability. Gives it billions of dollars. Makes Americans take its product. But won’t let you see the data supporting its product’s safety and efficacy. Who does the government work for?”
Aaron Siri
- The FDA managed to consider all 329,000 pages of data and grant emergency approval of the Pfizer vaccine within just 108 days, but asked for 55 years, and now upped that to 75 years to fully release that information to the public.
- After back and forth in court, on January 6, 2022 a federal court in the Northern District of Texas ordered the expedited release of all Pfizer documents relied upon by the FDA to authorise their COVID-19 vaccine. – READ
November 17, 2021 – Israel National News: FDA report finds all-cause mortality higher among vaccinated – FDA report shows Pfizer’s clinical trials found 24% higher all-cause mortality rate among the vaccinated compared to placebo group. Report emphasizes that “None of the deaths were considered related to vaccination.” – READ,
FDA Cominarty licencing report – HERE
- FDA fully approved COMINARTY in August 2021 with knowledge of this information:
- The report emphasized that “None of the deaths were considered related to vaccination.”
- A follow-up assessment of trial participants (tmt and placebo) completed on March 13, 2021 looked at the overall health outcomes of the trial participants which shows a significantly higher number of all-cause fatalities among the vaccinated cohort.
- Pfizer’s intrem report in July 2021 showed 15 deaths among the nearly 22,000 vaccine recipients, versus 14 deaths among the nearly 22,000 placebo recipients – only 3 related to COVID-19, one in vaccine group.
- FDA’s report Nov 2021 revealed update of 17 deaths among the control group and 21 in the vaccinated cohort, a relative difference of 23.5%.

November 16, 2021 – WHO | OCHA Relief Web: Pfizer, BioNTech and Moderna making $1,000 profit every second while world’s poorest countries remain largely unvaccinated – READ, ARCHIVE, CREDIT, WHO OCHA – WIKI
- “Based on company financial statements, the Alliance estimates that Pfizer, BioNTech and Moderna will make pre-tax profits of $34 billion this year between them,” which works out to $93.5 million profit per day
November 13, 2021 – Reuters: Fact Check-Pfizer is not including tromethamine in its COVID-19 vaccine to counter side-effects – READ , but it is included with known side effects – GETTR
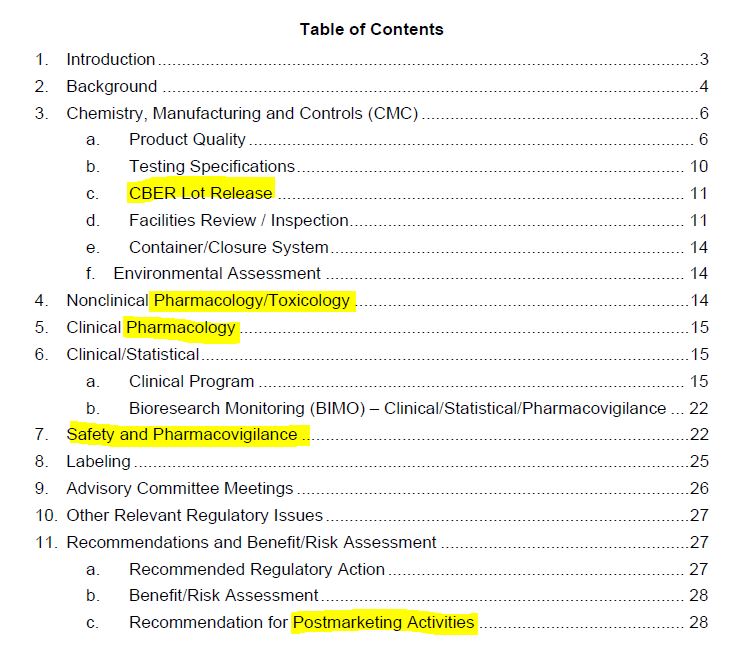
November 8, 2021 – FDA “Summary Basis for Regulatory Action” for Cominarty = FDA’s approval of BioNTech Manufacturing GmbH (in partnership with Pfizer, Inc.) Biological License Application (BLA) – READ, Karen Kingston – CREDIT
- “What EUA mRNA vaccine lots are the same as the FDA approved formulation of COMIRNATY?” Pfizer responded: “Here’s a list of lot numbers for EUA mRNA vaccines that are the same formulation as COMIRNATY.”
- “This means that throughout 2021, there were some Pfizer EUA mRNA lots that had the same formulation as COMIRNATY and a whole bunch of lots that had different mRNA formulations.” – REF
November 4, 2021 – NEJM: Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine through 6 Months – Thomas et al – READ, PDF, critical review – WATCH – Originally published September 15, 2021 – ARCHIVE
November 3, 2021 – Pfizer whistleblower Brook Jackson exposes multiple issues with Pfizer’s Covid-19 vaccine trial – READ, REPORT
November 2, 2021 – PRESS RELEASE: PFIZER REPORTS THIRD-QUARTER 2021 RESULTS – $24.1 Billion, Reflecting 130% Operational Growth – READ
November 2, 2021 – British Medical Journal (BMJ): Covid-19: Researcher blows the whistle on data integrity issues in Pfizer’s vaccine trial – Brook Jackson story by Paul D Thacker – READ & WATCH
October 29, 2021 – FDA Press Release: FDA Authorizes Pfizer-BioNTech COVID-19 Vaccine for Emergency Use in Children 5 through 11 Years of Age – READ, ARCHIVE
October 28, 2021 – PRESS RELEASE: Pfizer and BioNTech to Provide U.S. Government an Additional 50 Million Pediatric Doses of COVID-19 Vaccine to Support Further Preparedness for Future Needs – READ
October 26, 2021 – VRBPAC – FDA Briefing Document – EUA amendment request for Pfizer-BioNTech COVID-19 Vaccine for use in children 5 through 11 years of age – READ, meeting TRANSCRIPT
- Pfizer requests a formulation change to include “tromethamine (Tris) buffer” (with no clinical trial information):
- [Tromethamine, also known as Tris = Tris(hydroxymethyl)aminomethane or THAM – GETTR]
October 6, 2021 – NEJM – Waning of BNT162b2 Vaccine Protection against SARS-CoV-2 Infection in Qatar by Chemaitelly et al – READ, The Highwire – CREDIT – see vaccine effectiveness
October 6, 2021 – National Pulse: REVEALED: Pfizer Lobbying Hits Decade High as DOZENS of High-Profile Political Appointees Become Big Pharma Reps – [ Pfizer & Moderna names] – READ
- “Many of the new Big Pharma hires have come from consulting firms with deep and historical links to the current White House, and President Joe Biden himself.
- Pfizer declared lobbyists:
- 2019 = 77
- 2020 = 102
- 2021 = 92 (so far)
October 4, 2021 – National Pulse: COVERT VIDEO: Self-Declared “Evil” Pfizer Scientists Admit Natural Immunity BETTER Than Vaccine… ‘Our Org is Run on COVID Now’ – Hidden camera footage released by Project Veritas – WATCH & READ
- Scientists have labelled their own pharmaceutical company, Pfizer, “evil” on
- Scientists admitted that natural immunity rather than the vaccine induced immunity is more effective against COVID-19
- “our organization is run on COVID money now.”
October 1, 2021 – Chest: Myocarditis linked to Pfizer-BioNTech COVID-19 vaccine by Singh et al – READ
September 28, 2021 – Gen Bank: Synthetic construct HCV1147 Pfizer-BioNTech (BTN162b2) SARS-CoV-2 vaccine sequence – READ, The Pfizer vaccine genetic code is “tagged”, can be identified by PCR, clinical trial consequences???!!!! – JikkyLeaks – TWEET
- “Pfizer-BioNTech (BTN162b2) SARS-CoV-2 vaccine sequence; detected in patient plasma day 15 after dose 1″
September 26, 2021 – Pfizer CEO: “Normal Life” Won’t Return Without Regular COVID Vaccinations: – Albert Bourla, the CEO of the pharmaceutical giant Pfizer told ABC’s George Stephanopoulos, normal life will return within a year, but not for those who don’t have regular COVID-19 vaccinations. – READ, WATCH
September 23, 2021 – The Highwire Ep 234: ICAN DEMANDS PFIZER REVEAL VACCINE INGREDIENT – ICAN’S legal team has demanded Pfizer disclose a redacted ingredient on their Covid19 vaccine excipient, an ingredient that makes up 20% of their vaccine. – WATCH
September 22, 2021 – PRESS RELEASE: Pfizer and BioNTech Expand Collaboration with U.S. to Provide 500 Million Additional COVID-19 Vaccine Doses at Not-for-Profit Price for Donation to Poorest Countries – READ
September 20, 2021 – Judicial Watch, the government watchdog group, announced today that it filed a Freedom of Information Act (FOIA) lawsuit against the Department of Health and Human Services (HHS) for biodistribution studies and related data for the Pfizer, Moderna, and Johnson & Johnson vaccines – READ
- FDA, CDC and NIAID failed to respond to a June 7, 2021, FOIA request for:
- “[A]ccess to biodistribution studies and related data for the Pfizer, Moderna, and Johnson & Johnson vaccines used to treat and/or prevent SARS-CoV-2 and/or COVID-19.”
September 15, 2021 – Clinical trial 6 month data first published – ARCHIVE, now wee November 4, 2021 for updated version
September 15, 2021 – NEJM: Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine through 6 Months – Thomas et al (C4591001 Clinical Trial Group) – READ
September 8, 2021 – The Telegraph: Pfizer aims to submit trial data for children aged five to 11 ‘this month’ – The pharma giant’s chief executive added that the jab works ‘very well’ in children, amid mounting debates in the UK around vaccinating kids – READ (This was found to be misleading – HERE)
September 3, 2021 – News Punch: Pfizer Is Developing ‘Covid Pills’ – Pfizer and Merck & Co Inc announced on Wednesday new trials of their experimental oral antiviral drugs for COVID-19. – READ
September 1, 2021 – Corey’s Digs: The FDA Bait-and-Switch on Pfizer Covid-19 Jab – READ
August 30, 2021 – ACIP Committee – C4591001 COVID-19 BLA Safety and Efficacy Data For ACIP – CDC presentation – READ, ARCHIVE,
- C4591001 COVID-19 BLA Safety and Efficacy Data for ACIP presented by John Perez, VP Pfizer Vaccine Clinical R&D – PDF
August 26, 2021 – Featured News: Media Executive at Reuters Is Board Member of Pfizer – READ, TWEET, CREDIT James Smith Pfizer Board – HERE
August 25, 2021 – PRESS RELEASE: Pfizer and BioNTech Initiate Rolling Submission of Supplemental Biologics License Application to U.S. FDA for Booster Dose of COMIRNATY® in Individuals 16 and Older – READ
- New Phase 3 data show booster (third) dose of COMIRNATY induces significant SARS-CoV-2 neutralizing antibody titers and demonstrated a favorable safety and tolerability profile
- SARS-CoV-2 neutralizing titers against the wild-type strain one month after booster dose were 3.3 times the titers one month after the second dose
- Pfizer and BioNTech intend to file these data with the European Medicines Agency (EMA) and other regulatory authorities around the world in coming weeks
August 24, 2021 – CHD: 2 Things Mainstream Media Didn’t Tell You About FDA’s Approval of Pfizer Vaccine – READ, Dr Nass – READ, Pfizer Approval Letter (Archive) – PDF
- Buried in the fine print of the BLA approval letter by the FDA for the Pfizer Comirnaty COVID-19 vaccine are two critical facts that affect whether the vaccine can be mandated, and whether Pfizer can be held liable for injuries.

August 23, 2021 – CDC: COVID-19 Vaccine Related Codes & Crosswalk – includes table showing Pfizer/BioNTech – BLA-licensed (16+) – ARCHIVE,
- “COMINARTY products are not orderable at this time. NDCs are listed per FDA Structured Product Label (SPL) document for the BLA licensed product. These codes are not included in CDC Vaccine Code Set files at this time. Pfizer has provided the following statement regarding the COMINARTY branded NDCs and labels: “Pfizer received FDA BLA license on 8/23/2021 for its COVID-19 vaccine for use in individuals 16 and older (COMIRNATY). At that time, the FDA published a BLA package insert that included the approved new COVID-19 vaccine tradename COMIRNATY and listed 2 new NDCs (0069-1000-03, 0069-1000-02) and images of labels with the new tradename. At present, Pfizer does not plan to produce any product with these new NDCs and labels over the next few months while EUA authorized product is still available and being made available for U.S. distribution. As such, the CDC, AMA, and drug compendia may not publish these new codes until Pfizer has determined when the product will be produced with the BLA labels.”” – PDF
- Links to AMA: COVID-19 CPT vaccine and immunization codes : New Current Procedural Terminology (CPT®) codes have been created that streamline the reporting of immunizations for the novel coronavirus (SARS-CoV-2, also known as COVID-19) – (Sep 29, 2021)- ARCHIVE
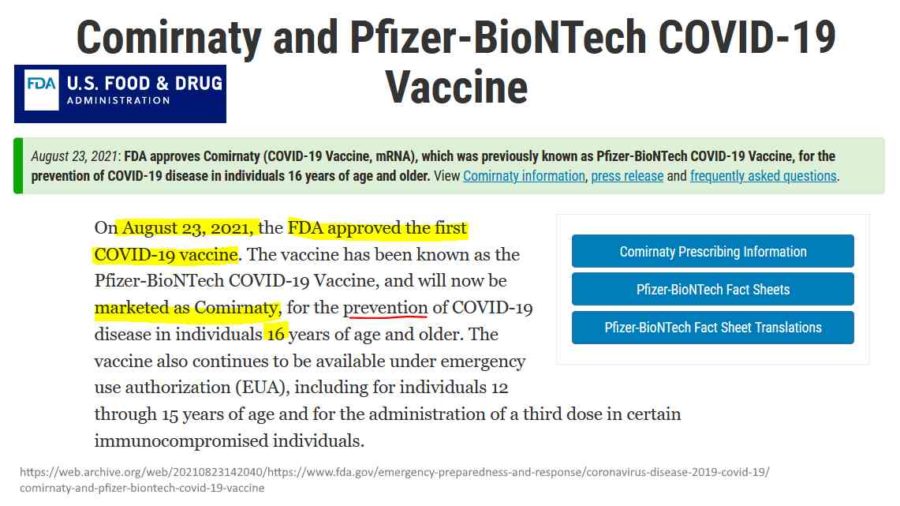
August 23, 2021 – NY Post: FDA grants full approval to the Pfizer-BioNTech COVID vaccine – READ
August 21, 2023 – FDA: Comirnaty and Pfizer-BioNTech COVID-19 Vaccine – ARCHIVE
- Includes links to Comirnaty Prescribing Information, Fact Sheets, Regulatory Information etc
August 23, 2021 – FDA: FDA approved Biologics License Application (BLA) for Pfizer’s COMIRNATY- PRESS, CDC – CODES, ARCHIVE, FDA grants full approval for Pfizer-BioNTechs COVID-19 vaccine called COMIRNATY- Approval LETTER, Official FDA COMIRNATY – FDA, Dr Anthony Fauci’s comments re BLA – WATCH, TIMELINE, James Corbett – WATCH, NBC – WATCH
…we recognize that for some, the FDA approval of a vaccine may now instill additional confidence to get vaccinated.
Acting FDA Commissioner Janet Woodcock, M.D.
- It is soon found out that they will not supply the branded (non-EUA) product until 2023!
August 23, 2021 – FDA “Summary Basis for Regulatory Action” for BLA of COMIRNATY – ARCHIVE, FDA updates the same file name, to see all BLA updates visit archives – HERE


August 23, 2021 – FDA News Release: FDA Approves First COVID-19 Vaccine – Approval Signifies Key Achievement for Public Health – READ, ARCHIVE, Dr Malone comment – EXCERPT, WATCH
- “There are now TWO LEGALLY distinct (Pfizer vs. BionTech), but otherwise identical products, based on two FDA letters, as well as a press release. The analysis of these FDA products below is preliminary and subject to change.” – Malone et al press release – READ, discussed by Dr Robert Malone – EXCERPT, WATCH
- The FDA press release (above): “The efficacy claims are based on outdated data” from alpha and beta variant ETC…
- Aug 23, 2021 LETTER 1 addressed to Pfizer “does not give full approval”, link titled “Pfizer-BioNTech COVID-19 Vaccine EUA LOA reissued August 23, 2021” – ARCHIVE
- Aug 23, 2021 -LETTER 2 addressed to BioNTech (COMIRNATY) – “BLA Approval” – ARCHIVE
August 13, 2021 – NY Post: FDA approves third vaccination shot for at-risk patients – BOOSTER EUA – READ
August 12, 2021 – FDA PRESS: Coronavirus (COVID-19) Update: FDA Authorizes Additional Vaccine Dose for Certain Immunocompromised Individuals – Other fully vaccinated individuals do not need an additional vaccine dose right now — Pfizer & Moderna – READ, ARCHIVE
August 3, 2021 – NY Times: F.D.A. Aims to Give Final Approval to Pfizer Vaccine by Early Next Month – READ
- The FDA move is expected to kick off more vaccination mandates for hospital workers, college students and federal troops
August 3, 2021 – NY Post: Feds look to speed up full approval of Pfizer vaccine – “The FDA is ramping up its timeline to fully approve Pfizer-BioNTech’s two-shot COVID-19 vaccine, amid the surge of infections gripping much of the US.” – READ, NY Times: “by early next month” – READ – See August 23, 2021!!
- “The Food and Drug Administration’s move is expected to kick off more vaccination mandates for hospital workers, college students and federal troops.” – REF
July 30, 2021 – STAT new: FDA, under pressure, plans ‘sprint’ to accelerate review of Pfizer’s Covid-19 vaccine for full approval – READ
July 28, 2021 – Forbes: Pfizer Expects $33.5 Billion In Vaccine Revenue In 2021 – READ
July 28, 2021 – MedRxiv prepring: Six Month Safety and Efficacy of the BNT162b2 mRNA COVID-19 Vaccine by Thomas et al – READ, PDF
- More people died who received the vaccine vs placebo: “Adding this up, in the clinical trial from July 2020 to March 2021, 20 deaths occurred among those who received the vaccine as compared to 14 who received the placebo.” – Aaron Siri – REF
July 23, 3021 – PRESS RELEASE: Pfizer and BioNTech to Provide U.S. Government with an Additional 200 Million Doses of COVID-19 Vaccine to Help Meet Continued Need for Vaccine Supply in the U.S. – READ
July 23, 3021 – Forbes: Pfizer shot just 39% effective against Delta infection, but largely prevents servere illness, Israel study suggests – READ, Dr Malone – WATCH
July 12, 2021 – NY Post: Pfizer, US health officials to discuss COVID boosters on Monday – Pfizer Inc will meet with federal health officials to discuss the need for a booster dose of the COVID-19 vaccine as it prepares to seek EUA for booster dose – “amid the spread of variants and data they said showed heightened risk of infection six months after initial inoculation.” – READ
July 9, 2021 – Clinical Trials.gov: To Evaluate the Safety, Tolerability, Efficacy and Immunogenicity of BNT162b2 Boosting Strategies Against COVID-19 in Participants ≥12 Years of Age – ClinicalTrials.gov Identifier: NCT04955626 – READ,
July 8, 2021 – NY Post: Fully vaccinated Americans ‘don’t need booster shot’ for Delta variant: CDC – Pushing back on Pfizer’s statement – READ
July 8, 2021 – NY Times: Citing the Delta Variant, Pfizer Will Pursue Booster Shots and a New Vaccine – Scientists were critical of the announcement, pointing to evidence that the current two-dose regimen is powerfully effective against the coronavirus – READ
- Pfizer announced they are developing a new vaccine version that “targets Delta”
- Pfizer and BioNTech also reported promising results from studies of people who received a third dose of the original vaccine. A booster given six months after the second dose of the vaccine increases the potency of antibodies against the original virus and the Beta variant by five- to tenfold, the companies said.
- “booster doses may be needed to fend off virus variants.” [is the leaky vaccine pressuring the virus to render variants more prevalent?]
- “The Delta variant, first identified in India, is believed to be about 60 percent more contagious than Alpha, the version of the virus that tore through Britain and much of Europe earlier” in 2021
July 8, 2021 – NY Post: Pfizer developing COVID booster shot to protect against Delta variant – READ
July 4, 2021 – Pfizer’s Shocking Cover Up of Maddie De Garay – WATCH
July 1, 2021 – NY Times guest opinion: It’s Time for the F.D.A. to Fully Approve the mRNA Vaccines by Dr. Topol – READ
June 25, 2021 – Epoch Times: FDA Adds Warning About Heart Inflammation to COVID-19 mRNA Vaccines [myocarditis] – READ, FDA update – READ
- “Today [June 25, 2021], the FDA is announcing revisions to the patient and provider fact sheets for the Moderna and Pfizer-BioNTech COVID-19 vaccines regarding the suggested increased risks of myocarditis (inflammation of the heart muscle) and pericarditis (inflammation of the tissue surrounding the heart) following vaccination.”
June 23, 2021 – ACIP met to discuss myocarditis post mRNA vaccination revealing alarming preliminary statistics in children and young adult – WATCH, TIMELINE, Discussed on The Highwire Ep 221 – EXCERPT
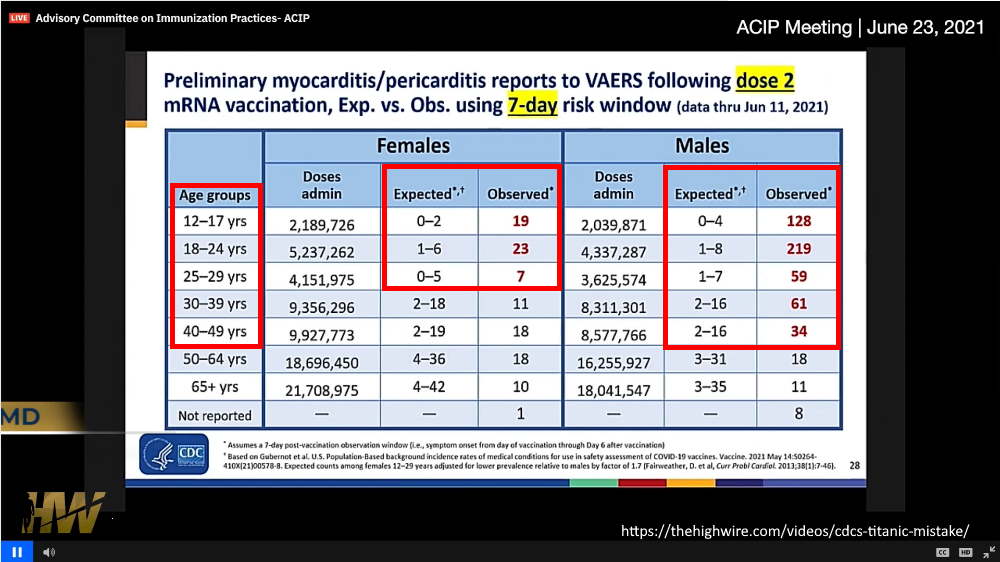
June 10, 2021 – PRESS RELEASE: Pfizer and BioNTech to Provide 500 Million Doses of COVID-19 Vaccine to U.S. Government for Donation to Poorest Nations – purchases at non-profit price – READ
May 28, 2021 – European Medicines Agency: First COVID-19 vaccine approved for children aged 12-15 in EU – Pfizer’s mRNA – READ
May 24, 2021 – TGA approves more flexible storage conditions for Pfizer-BioNTech COVID-19 vaccine – from 5 to now 31 days – ARCHIVE
- TGA received the application from Pfizer Australia Pty Ltd on Thursday May 20, 2021 – decision based on additional stability studies supplied by Pfizer
- “This change extends the approved storage period of the unopened thawed vial at 2-8°C (i.e. in a normal refrigerator after taking out of deep-freeze conditions) at the point of use from five days up to one month (31 days). The changed storage requirements will enable much greater flexibility in the distribution of the vaccine and have a significant impact on the roll out of the vaccine across Australia.”
- Note April 8, 2021 – “A storage condition of ‑20±5°C for up to 2 weeks (even during transportation) within the 6 month shelf life when stored at -90 to -60°C has been approved.” – REF
May 24, 2021 – Morgan C Jonas: Pfizer document suggests potential of vaccine transmission – WATCH
- TGA updated it’s definition of medical device on Aug 25, 2020 to now included “software, implant, reagent”!
May 19, 2021 – FDA: FDA Authorizes Longer Time for Refrigerator Storage of Thawed Pfizer-BioNTech COVID-19 Vaccine Prior to Dilution, Making Vaccine More Widely Available – authorised: “refrigerator at 2°C to 8°C (35°F to 46°F) for up to 1 month. Previously, thawed, undiluted vaccine vials could be stored in the refrigerator for up to 5 days” making the vaccine more widely available – READ
May 17, 2021 – Government of Canada: Producing lipid nanoparticle technology for vaccine delivery (Acuitas Therapeutics) – for Pfizer COVID-19 vaccine – READ
May 7, 2021 – NY Post: Pfizer-BioNTech seeks full FDA approval for COVID-19 vaccine – “The companies are the first COVID vaccine makers in the nation to apply for full approval, called a biologics license application, which would allow Pfizer to market the vaccine directly to consumers.” READ
May 4, 2021 – NY Post: Pfizer expects $26 billion in 2021 sales from COVID vaccine alone – READ
April 24, 2021 – Reuters: EU to shortly sign world’s largest vaccine deal with Pfizer – 1.8 billion doses – READ, The Highwire CREDIT
- European Commission President Ursula von der Leyen negotiated the deal with Pfizer CEO via text message! – READ, READ, But when challenged whe can’t find the texts – READ
- In April 2021, the New York Times first reported on text messages exchanged – READ
April 23, 2021 – Israel Times: Israel said probing link between Pfizer shot and heart problem in men under 30 -Leaked Health Ministry probe raises concerns as 62 cases of myocarditis found out of 5 million vaccinated — most after second dose; 2 deaths, but no direct link established – READ, ARTICLE, ARTICLE2
- The report was authored by senior ministry officials led by Prof. Dror Mevorach, head of one of the COVID-19 units at Hadassah Hospital Ein Kerem
April 15, 2021 – CNBC: Pfizer CEO says third Covid vaccine dose likely needed within 12 months – READ – Recording from April 1, 2021 – READ,
“A likely scenario is that there will be likely a need for a third dose, somewhere between 6 and 12 months and then from there, there will be an annual re-vaccination, but all of that needs to be confirmed.”
April 8, 2021 – TGA: Approval of updated storage conditions for the Pfizer COVID-19 vaccine – ARCHIVE, Comirnaty label – ARCHIVE
- “A storage condition of ‑20±5°C for up to 2 weeks (even during transportation) within the 6 month shelf life when stored at -90 to -60°C has been approved.”
- “In addition, unopened vials can now be stored for up to 5 days at domestic refrigerator temperatures (2°C to 8°C). Within this 5 day period, up to 12 hours may be used for transportation, but the time used for transport of unopened vials at refrigerator temperatures counts against the 5 day limit for storage at 2°C to 8°C. Once thawed, COMIRNATY should not be re-frozen.”
- COMIRNATY is diluted with saline prior to administration. The diluted vaccine, in either vials or syringes can be stored or transported at room temperatures of up to 30°C for up to 6 hours. The preparer of the reconstituted vaccine must be careful to manage microbiological risks and package integrity, particularly for prepared dosing syringes.”
April 8, 2021: Wider storage and transportation conditions for the Pfizer COVID-19 vaccine now approved – ARCHIVE
- “While longer term storage at dry ice or ultra-cold temperatures (-90C to -60°C) is still required, for unopened vials, storage and transportation at domestic freezer temperatures (-25°C to -15°C) is now permitted for up to 2 weeks.”
April 2, 2021 – ABC News au: Pfizer says COVID-19 shot is 91 per cent effective in updated data, protective against South African variant – READ
April 1, 2021 – NY Post: Pfizer says COVID-19 vaccine lasts 6 months, protects against variants – READ
April 2, 2021 – Reuters: Pfizer says its vaccine is effective against South African variant – READ
- The shot was also 100% effective in preventing illness among trial participants in South Africa – in 9 people!
April 1, 2021: PRESS RELEASE UPDATE PHASE III CLINICAL TRIAL: Pfizer and BioNTech Confirm High Efficacy and No Serious Safety Concerns Through Up to Six Months Following Second Dose in Updated Topline Analysis of Landmark COVID-19 Vaccine Study – READ, ALT
- Vaccine was 100% effective in preventing severe disease as defined by the U.S. CDC and 95.3% effective in preventing severe disease as defined by the FDA
- Vaccine was 100% effective in preventing COVID-19 cases in South Africa, where the B.1.351 lineage is prevalent
March 26, 2021 – PRESS RELEASE: EMA Approves New Storage Option for Pfizer-BioNTech Vaccine, Easing Distribution and Storage of Doses Across European Union – READ
March 11, 2021 – PRESS RELEASE: Real-World Evidence Confirms High Effectiveness of Pfizer-BioNTech COVID-19 Vaccine and Profound Public Health Impact of Vaccination One Year After Pandemic Declared – Isreal MOH data – READ, ALT
March 8, 2021 – Very Well Health: New Storage Guidance Means More Pfizer Vaccine Availability – The FDA is allowing the Pfizer-BioNTech COVID-19 vaccine to be stored at normal freezer temperatures instead of in ultra-cold freezers, Once thawed and diluted, the vaccine has the same shelf life of five days before degradation makes it ineffective.- READ
March 5, 2021 – New York Times via Ekathimerini: How Pfizer makes its Covid-19 vaccine – READ
- Raw material: Trillions of genetically engineered Escherichia coli bacteria produce DNA plasmids (small rings of DNA) containing coronavirus genes [spike?] – the raw material for the Pfizer-BioNTech vaccine.
- Pull raw material DNA from master cell bank cold storage – –150oC (–238F)
- Grow the modified E.coli containing “spike” plasmids
- Ferment the mixture – 300 liters vats making trillions of copies of the DNA plasmids
- Harvest and purify the DNA – add chemicals to break open the bacteria and release the plasmids, purify to remove the bacteria debris
- Test for quality – ensure plasmid gene sequence is unchanged
- Cut the plasmids using enzymes (linearization)
- Filter the DNA
- Freeze, pack and ship
- Transcribe the DNA into mRNA – yielding 7.5 million doses /batch
- Test the mRNA
- Freeze, pack and ship (again)
- Prepare the mRNA
- Prepare the lipids – cationic lipids
- Assemble the mRNA vaccine – create lipid nanoparticles – electric charge pulls them together in a nanosecond, encapsulating the mRNA
- Prepare the vials
- Rush to fill the vials – 575 vials/min @ 0.45mL concentrate/vial, enough for 6 doses after dilution.
- Package, freeze and test
- Pack and ship the finished vaccine
February 26, 2021 – NBC News: Exclusive Interview With Pfizer CEO Albert Bourla – possibility of a third booster shot and the progress of their pediatric vaccine trials – WATCH
February 25, 2021 – FDA PRESS RELEASE: FDA Allows More Flexible Storage, Transportation Conditions for Pfizer-BioNTech COVID-19 Vaccine -allowing undiluted frozen vials of the Pfizer-BioNTech COVID-19 Vaccine to be transported and stored at conventional temperatures commonly found in pharmaceutical freezers for a period of up to two weeks. – READ
February 24, 2021 – LA Times: Pfizer’s vaccine trial data holds up in the real world, according to large-scale study in Israel – READ
Febraury 23, 2021 – The West Australian: More Pfizer jab doses arrive in Australia – READ
(Australia imported all their COVID-19 vaccines)
February 19, 2021 – Reuters: Israeli study finds Pfizer vaccine 85% effective after first shot – READ,
- Sheba Medical Center’s findings …”among 7,214 hospital staff who received their first dose in January, there was an 85% reduction in symptomatic COVID-19 within 15 to 28 days. The overall reduction of infections, including asymptomatic cases detected by testing, was 75%.” The “cohort studied at the hospital were “mostly young and healthy””
February 19, 2021 – Observer: Vaccine Breakthrough: Pfizer COVID-19 Vaccine No Longer Requires Deep Freezing – New data submitted to FDA suggest that Pfizer’s vaccine can be stored at normal freezer temperatures for up to two weeks. – READ
- Current EUA requires storage at ultra-cold temperatures between -80ºC and -60ºC (-112ºF to ‑76ºF) at all times.
- New “stability data generated on vials manufactured over the past nine months showed that the vaccine can be stored at -25°C to -15°C (-13°F to 5°F), temperatures for up to two weeks.
February 19, 2021 – PRESS RELEASE FREEZER STABILITY: Pfizer and BioNTech Submit COVID-19 Vaccine Stability Data at Standard Freezer Temperature to the U.S. FDA – READ, ARCHIVE,
- NY Post: Pfizer says its COVID vaccine doesn’t need to be stored at ultra-freezing temps anymore – ARTICLE Sub-zero temp. are a logistical nightmare – READ, Moderna’s mRNA vax only required normal freezing temps – ARCHIVE
February 15, 2021 – Aust PM Scott Morrison Media Release: First Pfizer Vaccine Doses Arrive in Australia – ARCHIVE
February 15, 2021 – Pfizer PRESS RELEASE: Pfizer and BioNTech Commence Global Clinical Trial to Evaluate COVID-19 Vaccine in Pregnant Women – READ, ARCHIVE, The Highwire – WATCH, Maryanne Demasi – READ
- “The Phase 2/3 trial is designed as a randomized, placebo-controlled, observer-blind study in approximately 4,000 healthy pregnant women 18 years of age or older vaccinated during 24 to 34 weeks of gestation.” Only late stage pregnancy
- Clinical trial identifier NCT04754594 “To Evaluate the Safety, Tolerability, and Immunogenicity of BNT162b2 Against COVID-19 in Healthy Pregnant Women 18 Years of Age and Older” – READ
- Est. trial participants went from 4000 only 350: 4000 participants Feb 2021 – ARCHIVE, 700 Aug 2021 – ARCHIVE, 700 not recruiting – ARCHIVE, ONLY 350 May 19, 2022 – ARCHIVE
- “This will be a Phase 2/3, randomized, placebo-controlled, observer-blind study evaluating the safety, tolerability, and immunogenicity of 30 µg of BNT162b2 or placebo administered in 2 doses, 21 days apart, in approximately 350 healthy pregnant women 18 years of age or older vaccinated at 24 to 34 weeks’ gestation.”
- July 2023 – “Eventually 348 maternal participants were randomized to receive treatment. 335 were infants bort to maternal participants.” Leaving 13 pregnancies unaccounted for – an underpowered study, – REF
- They are removing the placebo group, so no long term data on an already under pwered study!!!
January 29, 2021 – PRESS RELEASE: Pfizer and BioNTech Publish Data on COVID-19 Vaccine-Induced Antibodies’ Ability to Neutralize SARS-CoV-2 U.K. Strain Pseudovirus in Cell Culture in Science – READ
January 27, 2021 – PRESS RELEASE: In Vitro Studies Demonstrate Pfizer and BioNTech COVID-19 Vaccine Elicits Antibodies that Neutralize SARS-CoV-2 with Key Mutations Present in U.K. and South African Variants – READ
January 15, 2021 – International Federation of Pharmaceutical Manufacturers and Associations (IFPMA) Press Release: Biopharmaceutical global body kicks off 2021 with new CEO leadership line up – READ, Leadership – HERE
- Albert Bourla is appointed IFPMA Vice President and serve as the Chair and Vice Chairs of the IFPMA CEO Steering Committee
January 22, 2021 – PRESS RELEASE: Pfizer and BioNTech Reach Agreement with COVAX for Advance Purchase of Vaccine [40M doses in 2021] to Help Combat COVID-19 – READ
January 22, 2021 – PRESS RELEASE – Global News Wire: Ten Leading BioPharma Companies Announce Formation of Accumulus Synergy to Develop Global Data Sharing Platform – READ
- Companies include Pfizer, GSK, Janssen, Lilly, Roche, Sanofi and Takeda etc
- Rollout of a cloud-based platform for the biopharma industry communicate and exchanges information with health authorities worldwide.
January 20, 2021 – PRESS RELEASE: Pfizer and BioNTech Publish Results of Study Showing COVID-19 Vaccine Elicits Antibodies that Neutralize Pseudovirus Bearing the SARS-CoV-2 U.K. Strain Spike Protein in Cell Culture – READ
January 15, 2021 – PRESS RELEASE: Statement by Pfizer Chairman and CEO Albert Bourla – President-elect Biden released a COVID-19 vaccination plan that our scientists reviewed and believe will accelerate our ability to beat back the pandemic and save American lives. – READ
January 8, 2021 – PRESS RELEASE: An In Vitro Study Shows Pfizer-BioNTech COVID-19 Vaccine Elicits Antibodies that Neutralize SARS-CoV-2 with a Mutation Associated with Rapid Transmission – READ
January 6, 2021 – “The Real World Epidemiological Evidence Collaboration Agreement” between Pfizer and Israel – ARCHIVE
- Israel is used as a pseudo “clinical trial” for Pfizer’s mRNA COVID-19 vaccine- TIMELINE
- January 7, 2021 Israel’s Prime Minister Benjamin Netanyahu announced his Operation Getting Back to Life deal with Pfizer Chairman and CEO Albert Bourla to exclusively use Pfizer’s COVID-19 vaccine product to vaccinate “all citizens of Israel over the age of 16 by the end of March and perhaps even earlier…” Making Israel “a global model state for the rapid vaccination of an entire country.” “We can do this because our health system is among the most advanced in the world”, being highly-digitalised. – READ, ARCHIVE
January 2021 – TGA: Nonclinical Evaluation Report – BNT162b2 [mRNA] COVID-19 vaccine (COMIRNATY) – Submission No: PM-2020-05461-1-2 (obtained under Freedom of Information March 2023? has redactions) – PDF, Aust. Senator Rennick – WATCH, BACKUP, READ, Dr John Campbell more – WATCH, Senator Rennick’s website more FOI requests – HERE
- JikkyLeaks on Twitter – see PHARMACOKINETICS – distribution Table 4-2 pg 45 accumulation of LNP in the ovaries after 48 hrs- TWEET
- From this DISTURBING data in the hands of the Australian regulator TGA, and surely the US FDA, should have been enought to NOT grant approval for the COVID-19 vaccine, at a minimum the public should have been informed – WATCH
- Page 4: “Almost similar microscopic lung inflammation was observed in both challenged control and immunised animals (macaques) after the peak of infection (Days 7/8)”
- Page 4: “There are no distribution and degradation data on the S antigen-encoding mRNA.”
- Page 5: “Antibodies and T cells in monkeys declined quickly over 5 weeks after the second dose of BNT162b2 (V9), raising concerns over long term immunity”
- Page 6: Many Unknowns identified by the TGA
- lack of pharmacokinetic data of mRNA code
- lack of repeat dose toxicity studies in a second species
- no genotoxicity studies with the novel excipients
- lack of studies investigating potential for autoimmune diseases
- Page 8: BNT162b2 immunisation also induced proinflammatory cytokines…
- Page 9: “One study found that among people who had recovered from COVID-19, 100% had S protein-specific CD4+ T cells in the circulation and 70% had S protein-specific CD8+ T cells in the circulation” [Natural infection confers strong protection!]
January 2021 – Ugur Sahin BioNTech: Next Generation Immunotherapy Investor presentation – slide (Dated Nov 10, 2020) PDF, CREDIT
- BioNTech is in partnership with Pfizer and Fosun Pharma (CCP) for development and commercialisation of COVID-19 Vaccines

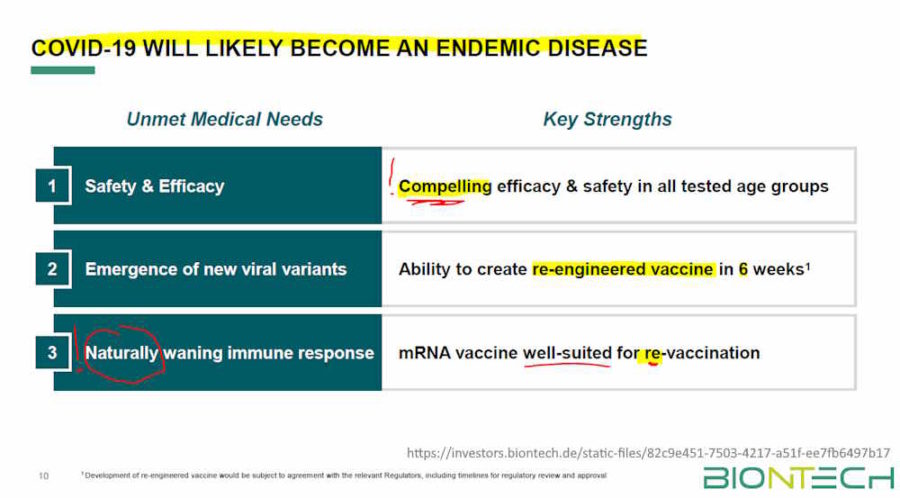
(Before Pfizer FOIA documents were released! Bold claims)
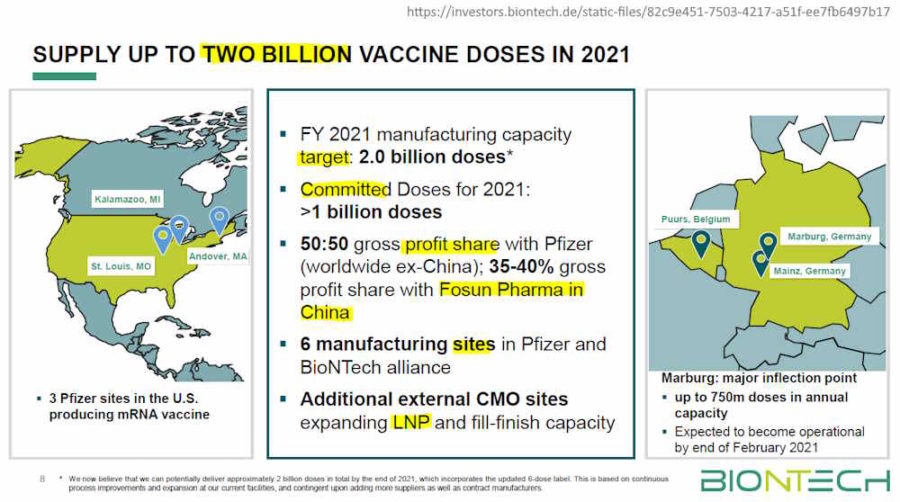
2020
December 31, 2020 – NEJM: Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine – Polack et al – READ, critical review – WATCH , Arkmedic Substack – “the 95% is false” – READ
- “A two-dose regimen of BNT162b2 conferred 95% protection against Covid-19 in persons 16 years of age or older. Safety over a median of 2 months was similar to that of other viral vaccines.” ClinicalTrials.gov Identifier: NCT04368728
- This clinical trial provides a “practical demonstration that RNA-based vaccines, which require only viral genetic sequence information to initiate development, are a major new tool to combat pandemics and other infectious disease outbreaks.” – AKA a new “vaccine” PLATFORM
- “The continuous phase 1/2/3 trial design may provide a [NEW] model to reduce the protracted development timelines that have delayed the availability of vaccines against other infectious diseases of medical importance.”
The Timline of events is concerning:
- December 10, 2020 – NEJM Phase 3 clinical trail results paper was published
- December 10, 2020 – Pfizer-BioNTech submission to the FDA’s VRBPAC committee was presented
- The Pfizer BNT162b2 GTV was “approved” December 11, 2020, “with no attempt to assess the 350,000 pages of documentation that the FDA requested 55 years to release to the public” – REF, Pfizer FOIA Docs – HERE
December 23, 2020 – The Telegraph: Dancing doctors celebrate Israel vaccine roll out – The Sourasky Medical Centre in Tel Aviv began vaccinating patients on December 20, 2020 – READ, WATCH
- “More than 3000 people in Israel have died of coronavirus. The country currently has over 3000 new cases per day.”
December 19, 2020 – CDC slides – ACIP COVID-19 Vaccine Work Group: Anaphylaxis following mRNA COVID-19 vaccine receipt – Pfizer – ARCHIVE
December 17, 2020 – NEJM: Safety and Immunogenicity of Two RNA-Based Covid-19 Vaccine Candidates – Walsh et al [Pfizer BNT162b1 and BNT162b2 vaccine candidates Phase 1 trail]- READ, Trial protocol – PDF
December 15, 2020 – The Highwire: FDA & MANUFACTURERS TO TERMINATE VACCINE SAFETY MONITORING – The FDA has revealed Pfizer, and other Covid vaccine makers, plan to unblind their Phase 2/3 placebo groups and vaccinate them after just six months, making it impossible to evaluate the long-term effects of this brand new vaccine platform – WATCH, TIMELINE
VRBPAC meeting on Dec 10, 2020 –
“Considerations for Placebo-Controlled Trial Design If An Unlicensed Vaccine Becomes Available” – SLIDES
FDA BRIEFING DOCUMENT – Sponsor’s intent to unblind placebo group after 6 months – EXCERPT, SOURCE
December 15, 2020 – CNBC: Pfizer CEO Albert Bourla on vaccine hesitancy: ‘Trust science’ – WATCH, COMPILATION!
- Bourla had not yet taken the vaccine! He doesn’t want to “cut in line”, He says your decision not to vaccinate will likely affect the lives of others!
December 14, 2020 – Pfizer removes the Placebo Control arm (Unblinds) of their Phase III clinical trail, eliminating the long term safety study arm – IMAGE, SOURCE, TIMELINE
December 12, 2020 – FDA grants first Emergency Use Authorization (EUA) to Pfizer-BioNTech’s COVID-19 vaccine – TIMELINE
December 12, 2020 – CNN Health: FDA issues emergency use authorization for Pfizer/BioNTech Covid-19 vaccine – CNN provided information about the novel new vaccine – READ
December 11, 2020 – US News: FDA Grants Emergency Use Authorization for Pfizer Vaccine – The approval allows vaccine shipments to begin – READ, Other articles – HERE, HERE, HERE,
December 11, 2020 – WSJ: Pfizer, BioNTech Covid-19 Vaccine Is Authorized in the U.S. – READ
December 11, 2020 – PRESS RELEASE: Pfizer and BioNTech Celebrate Historic First Authorization in the U.S. of Vaccine to Prevent COVID-19 – READ
December 11, 2020 – FDA NEWS RELEASE: FDA Takes Key Action in Fight Against COVID-19 By Issuing Emergency Use Authorization for First COVID-19 Vaccine – Action Follows Thorough Evaluation of Available Safety, Effectiveness, and Manufacturing Quality Information by FDA Career Scientists, Input from Independent Experts – READ
“While not an FDA approval, today’s emergency use authorization of the Pfizer-BioNTech COVID-19 Vaccine holds the promise to alter the course of this pandemic in the United States,”
Peter Marks, Director of the FDA’s Center for Biologics Evaluation and Research (CBER)
December 10, 2020 – The Telegraph: Israel may have to pause lightning vaccination programme as Pfizer stocks dwindle – More than 30 per cent of the over-60s have already been vaccinated – READ
December 10, 2020 – The Telegraph: The world’s fastest Covid inoculation drive: Israel vaccinates half a million in nine days – READ
December 10, 2022 – STAT News: FDA advisory panel endorses Pfizer/BioNTech Covid-19 vaccine – The vote: 17 Yes, 4 No, 1 abstention. “And with that, the FDA will very likely grant an emergency use authorization to the Pfizer/BioNTech Covid vaccine within days, if not sooner.” – ARTICLE
December 10, 2020 – FDA’s Vaccines and Related Biological Products Advisory Committee (VRBPAC) Meeting Announcement – to discuss Emergency Use Authorization (EUA) of the Pfizer-BioNTech COVID-19 Vaccine for the prevention of COVID-19 in individuals 16 years of age and older.- READ & LINKS, WATCH, FDA Press Release – READ
- The meeting is to answer this on question: “Based on the totality of scientific evidence available, do the benefits of the Pfizer-BioNTech COVID-19 Vaccine outweigh its risks for use in individuals 16 years of age and older?” – REF
- VRBPAC meeting presentation slides – HERE, ARCHIVE, Meeting attendees – HERE
- December 10, 2020 – VRBPAC meeting – BRIEFING DOCUMENT: PFIZER-BIONTECH COVID-19 VACCINE (BNT162, PF-07302048) – PDF1, PDF2, BACKUP, CREDIT
- “16.1.10 List of Laboratories Under Vaccine Research & Development Oversight” – Pearl River, NY laboratory – PDF, CREDIT
- Pfizer’s PRESENTATION: BNT162b2 Vaccine Candidate Against COVID-19 – SLIDES
- FDA Review of Efficacy and Safety of Pfizer-BioNTech COVID-19 Vaccine Emergency Use Authorization Request – SLIDES
December 10, 2020 – VRBPAC FDA Briefing Document: Pfizer-BioNTech COVID-19 Vaccine – PDF, ARCHIVE
- “Use in pregnancy and lactation and vaccine effectiveness are areas the Sponsor identified as missing information“
- “Available data do not indicate a risk of vaccine-enhanced disease, and conversely suggest effectiveness against severe disease within the available follow-up period. However, risk of vaccine-enhanced disease over time, potentially associated with waning immunity, remains unknown“
- FDA told manufacturers to look for vaccine associated enhanced disease aka ADE right before it issued the first EUA – CREDIT
December 9, 2020 – FDA News: Pfizer’s Vaccine Meets EUA Thresholds, Could Be Authorized This Weekend – An FDA Emergency Use Authorization (EUA) could come as soon as this weekend for Pfizer/BioNTech’s COVID-19 vaccine, with the vaccine advisory committee set to meet tomorrow to discuss the agency’s 53-page analysis concluding that the two-dose regimen met its strict EUA requirements – READ
December 8, 2020 – BBC: First Pfizer vaccine given to 90-year-old woman – Margaret Keenan, from Enniskillen, Coventry UK – READ, NBC News – READ, Manchester News – READ, Independent – READ
- Daily Mail: Matt Hancock breaks down in tears… William Shakespeare, 81, next in line – READ, worst acting award – READ, “Bill” died May 2021 – READ
- Second Dose 3 weeks later – The Guardian – READ, The Hill – READ
December 4, 2020 – WION: Pfizer CEO ‘not certain’ if coronavirus vaccine will prevent transmission – READ
- “Even though I’ve had the protection, am I still able to transmit it to other people?” NBC’s Lester Holt asked in an interview with Albert Bourla on December 3, 2020 “prompting a startling response from Bourla:
- ‘I think this is something that needs to be examined. We are not certain about that right now.” said Albert Bourla.
December 3, 2020 – Pharmacy Times: First Authorization in the World for a COVID-19 Vaccine Issued in UK for Pfizer and BioNTech Vaccine – READ
December 2, 2020 – Health Impact News: UK Government Warns Doctors About Infertility Possibility with Pfizer COVID Vaccine, But NO Warning to Patients! (sourced info) – READ, OTHER
December 2, 2020 – DW: UK approves Pfizer-BioNTech vaccine, to roll out next week – The UK will be the first country in the West to offer a COVID-19 vaccine to the public after a regulator approved the medicine in record time. – READ, TIMELINE
December 2, 2020 – CNN: First shipments of Pfizer vaccine to be delivered December 15, 2020 – according to Operation Warp Speed document provided to govenors – READ
November 20, 2020 – Biospace: The Whole World’s Watching: Pfizer-BioNTech COVID-19 Vaccine Data Heads to FDA Today – READ
- As expected after Pfizer and BioNTech announced their COVID-19 vaccine Phase III trial had completed and demonstrated a 95% efficacy rate, the companies plan to apply for an EUA with the FDA today.
November 18, 2020 – Science News: New Pfizer results show its COVID-19 vaccine is nearly 95% effective – READ
November 18, 2020 – PRESS RELEASE: Pfizer and BioNTech Conclude Phase 3 Study of COVID-19 Vaccine Candidate, Meeting All Primary Efficacy Endpoints – READ, ALT, ARCHIVE
“Primary efficacy analysis demonstrates BNT162b2 to be 95% effective against COVID-19 beginning 28 days after the first dose;170 confirmed cases of COVID-19 were evaluated, with 162 observed in the placebo group versus 8 in the vaccine group”

- [Note June 2023 from Pfizer FOIA documents show they dropped 200 vaccinated trial participants who contracted COVID-19 – WATCH, WATCH]
November 14, 2020 – NY Post: The modern-day Curies: Meet the scientist couple behind 90% effective COVID-19 vaccine [BioNTech’s Ozlem Tureci and Ugur Sahin] – READ
November 13, 2020 – WION: Volunteers face ‘severe hangover’, headache and pain after getting Pfizer vaccine shot – READ
November 10, 2020 – NY Post: Why Pfizer’s COVID-19 vaccine will be a nightmare to distribute – because of ultra-low storage temperatures – READ
November 10, 2020 – Newsmax: HHS Sec. Azar: Taking Pfizer At ‘Face Value’ on Vaccine Timing – READ
- HHS Director Alex Azar takes “Pfizer’s statements at “face value” that it released its data on its new vaccine almost a week after the presidential election”
- Trump “blasted Pfizer for the timing of its announcement, accusing it of not having the “courage” to make the announcement before the election.” – TWEET
- Pfizer was part of Operation Warp Speed – Azar – “A $2 billion contract that says they’re very much part”…”We assisted with ingredients, distribution, syringes, needles, dry ice for shipment, distribution planning. They’re very much part of Operation Warp Speed.” –
- Pfizer CEO had told NY Times “We were never part of the Warp Speed,” – NY Times – ARTICLE
November 9, 2020 – Newsmaz: Pfizer Denies Trump Admin Credit for Vaccine – READ, TWEET
November 9, 2020 – NY Times: Pfizer’s Early Data Shows Vaccine Is More Than 90% Effective – Pfizer announced stunning early results from its coronavirus vaccine trial, cementing the lead in a frenzied global race that has unfolded at record-breaking speed. – READ
- “The news comes just days after Joseph R. Biden Jr. clinched a victory over President Trump in the presidential election.”
- “Operation Warp Speed, the federal effort to rush a vaccine to market, has promised Pfizer $1.95 billion to deliver 100 million doses to the federal government…” [from July 22, 2020 – ARTICLE]
- “We were never part of the Warp Speed,”… “We have never taken any money from the U.S. government, or from anyone.”
November 9, 2020 – CBS News: Pfizer says trials show its COVID vaccine is “more than 90% effective” – READ, Fox biz – READ, The Week – READ
- “The vaccine proved to be more than 90% effective in the first 94 subjects [out of 44,000 total participants] who were infected by the new coronavirus and developed at least one symptom…Pfizer said it remains on track to collect at least two months of safety data during the third week of November and could file for an emergency authorization shortly thereafter.
- So far, no serious safety issues have arisen in the study, the companies said. The study has enrolled nearly 44,000 subjects in the U.S. and other countries.”
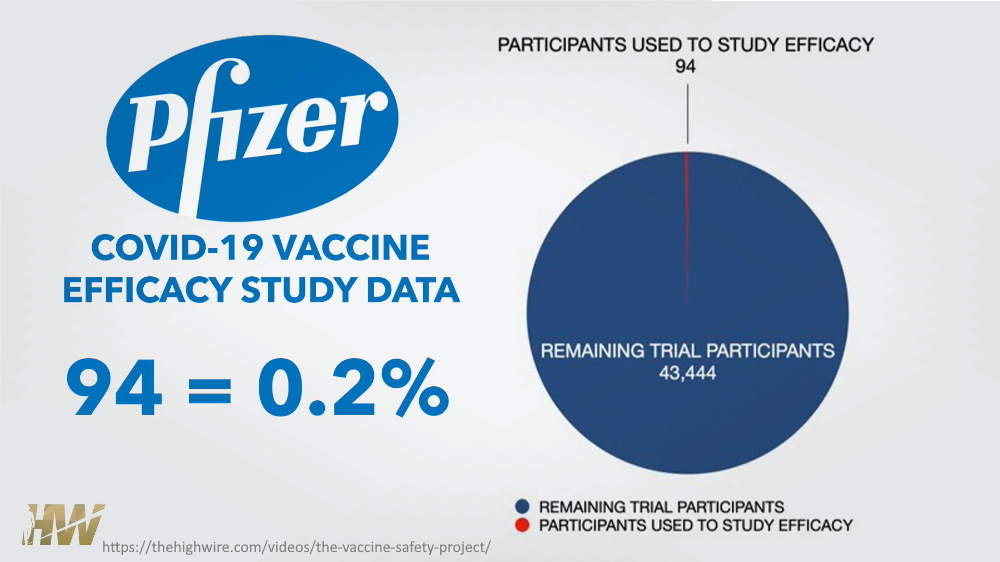
November 9, 2020 – Pfizer PRESS RELEASE PRELIMINARY PHASE 3 DATA: Pfizer and BioNTech Announce Vaccine Candidate Against COVID-19 Achieved Success in First Interim Analysis from Phase 3 Study – “Vaccine candidate was found to be more than 90% effective in preventing COVID-19” – READ, ARCHIVE
November 9, 2020 – BioNTech PRESS RELEASE: Pfizer and BioNTech Announce Vaccine Candidate Against COVID-19 Achieved Success in First Interim Analysis from Phase 3 Study – READ, ARCHIVE
- “Vaccine candidate was found to be more than 90% effective in preventing COVID-19 in participants without evidence of prior SARS-CoV-2 infection in the first interim efficacy analysis”
November 9, 2020 – Acuitas Therapeutics PRESS RELEASE: The BioNTech and Pfizer Positive COVID-19 Phase 3 Vaccine Data Comes with an Important Canadian Connection – The Lipid Nanoparticle delivery system – PDF, Acuitas – TIMELINE
October 16, 2020 – Pfizer CEO open letter announce their intention to apply to the US FDA for Emergency Use Authorisation in the third week of November 2020, once milestones are met. – LETTER, TWEET, ARTICLE
September 15, 2020 – PRESS RELEASE: Pfizer Investor Day Features Significant Number of Pipeline Advances for COVID-19 Programs and Across Numerous Therapeutic Areas – New stability, immunogenicity, and tolerability data for COVID-19 vaccine candidate, BNT162b2 – READ
July 27, 2020 – The Hill: Pfizer launches phase 3 trial of coronavirus vaccine with 30K participants – READ, [Be sure to check out Brook Jackson Pfizer trial whistleblower – HERE]. The same day Moderna begins Phase 3 – READ
July 27, 2020 – Pfizer begins Phase 3 double blind placebo controlled clinical trial – READ, Clinical trial number NCT04368728 (roll over of phase 1/2 trial) – ARCHIVE
- They state the trial was “designed to determine if the Pfizer-BioNTech COVID-19 vaccine is safe and effective in preventing COVID-19 disease.”. [note – It was not designed to test for the prevention of SARS-CoV-2 infection or transmission – of the pathogen. The latter might have a community benefit, the former only a potential personal benefit]
July 27, 2020 – PRESS RELEASE: Pfizer and BioNTech Choose Lead mRNA Vaccine Candidate Against COVID-19 and Commence Pivotal Phase 2/3 Global Study – READ , ARCHIVE [Start of Phase 2, which will roll into Phase 3 (larger group)]
- Nucleoside-modified messenger RNA (modRNA) candidate BNT162b2, which encodes an optimized SARS-CoV-2 full-length spike glycoprotein, at a 30µg dose level in a 2 dose regimen is advanced into Phase 2/3 Study
- Vaccine candidate and dose level was selected from “preclinical and clinical data obtained in Phase 1/2 US and German studies – Pfizer and BioNTech selected BNT162b2 as the candidate to progress to a Phase 2/3 study based on the totality of available data from our preclinical and clinical studies, including select immune response and tolerability parameters
- The Phase 2/3 study protocol follows all FDA guidance on clinical trial design for COVID-19 vaccine studies – READ, Clinical trial number NCT04368728
- “Trial regions to include areas with significant expected SARS-CoV-2 [the virus] transmission to assess whether investigational vaccine candidate, BNT162b2, is effective in preventing COVID-19 [the symptoms]”
July 22, 2020 – NY Times: Pfizer Gets $1.95 Billion to Produce Coronavirus Vaccine by Year’s End – for 100 million doses – READ
July 21, 2020 – From FOIA: DEPARTMENT OF THE ARMY -U.S. ARMY CONTRACTING COMMAND — NEW JERSEY -PICATINNY ARSENAL, NEW JERSEY 07806-5000 – PDF, CREDIT Military selects Pfizer
- “The Army Contracting Command — New Jersey (ACC-NJ), in supporting the Joint Project Manager — Medical Countermeasure Systems (JPM-MCS), issued MCDC RPP 20-11 on 09 June 2020. Members of the MCDC submitted proposals in accordance with this RPP. The Government received and evaluated all proposal(s) submitted and a Basis of Selection has been executed, selecting Pfizer, Inc. as the awardee.”
- MCDC = Medical CRBN Defense Consortium – WEBSITE, which works under the OTA
- RPP = Request for Prototype Proposals (put out by MCDC to members)
- JPM-MCS is part of the Joint Program Executive Office for Chemical and Biological Defense (JPEO-CBD) –REF
- Objective PRE-20-11 for “COVID-19 Pandemic — Large Scale Vaccine Manufacturing Demonstration”
- Pfizer is a “member” of MCDC
July 13, 2020 – PRESS RELEASE: Pfizer and BioNTech Granted FDA Fast Track Designation for Two [BNT162b1 and BNT162b2] Investigational mRNA-based Vaccine Candidates Against SARS-CoV-2 – ARCHIVE
- FDA Fast Track was granted based on preliminary data from Phase 1/2 studies (see July 1, 2020) for vaccine candidate BNT162b1 [NOT the final chosen candidate!]
- Anticipated large, global Phase 2b/3 safety and efficacy study may begin as early as July 2020
July 10, 2020 – Dr David Healy: Fernando Polack and Nicolás Vaquer, CEO of Pfizer in Argentina, called on President Fernandez to tell him that Argentina had been selected by Pfizer to conduct Phase III clinical trials of their Covid Vaccine – REF
- Jikkyleaks re clinical trial Sites 1231 and 4444 – CREDIT
- “With military precision “About 5,800 volunteers were enrolled, half getting the active vaccine. This is almost 4 times more than the next largest centre in this trial. Amazingly 467 doctors were almost instantly signed up and trained as assistant investigators in the study. Fernando was in command as Pfizer’s Principal Investigator.” – REF
- “Fernando is heavily involved through his Gates-sponsored Fundación INFANT in Buenos Aires in RSV trials and research.” [RSV is on the increase following the roll out of the mRNA jabs!]
- Vaccine injured Augusto Roux, volunteer number 12312982 in Pfizer’s Buenos Aires Covid Vaccine trial were Dr Polack was Principal Investigator – READ
July 1, 2020 – Pre-Print: Phase 1/2 Study to Describe the Safety and Immunogenicity of a COVID-19 RNA Vaccine Candidate (BNT162b1) in Adults 18 to 55 Years of Age: Interim Report – by Pfizer – ARCHIVE, published in Nature August 12, 2020 – READ, ClinicalTrials.gov identifier: NCT04368728 (April 30, 2020)
- A “placebo-controlled, observer-blinded dose escalation study among healthy adults, 18-55 years of age, randomized to receive 2 doses, separated by 21 days, of 10 μg, 30 μg, or 100 μg of BNT162b1 [not the final candidate?], a lipid nanoparticle-formulated, nucleoside-modified, mRNA vaccine that encodes trimerized SARS-CoV-2 spike glycoprotein RBD
- Local reactions and systemic events were dose-dependent, generally mild to moderate, and transient. RBD-binding
- IgG concentrations and SARS-CoV-2 neutralizing titers in sera increased with dose level and after a second dose
May 28, 2020 – IFPMA: Global Biopharma CEO/Top Executives COVID-19 Media Briefing – WATCH, SOURCE
Pfizer’s CEO, Albert Bourla, said his company doesn’t want funding from any government because “We believe we can move faster if we don’t have to involve a third party.”
May 7, 2020 – WebMD: Pfizer Starts Clinical Trials of COVID-19 Vaccine – Phase I/II – READ
April 30, 2020 – US Study: ClinicalTrials.gov identifier: NCT04368728 [Phase 1/2]: Study to Describe the Safety, Tolerability, Immunogenicity, and Efficacy of RNA Vaccine Candidates Against COVID-19 in Healthy Individuals (Sponsor = BioNTech SE, Collaborator = Pfizer, Study ID number = C4591001) – READ, ARCHIVE. Study completion date January 27, 2023, Trial Protocol – PDF
- “Informed by BNT162-01, [This] Phase 1/2 US study (C4591001) of the vaccine candidates started in May 2020.” –REF
- “Pfizer and BioNTech utilized this approach to efficiently optimize formulation and dose selection in the clinic. Study C4591001 is a single, multistage and multi-phase trial (including the pivotal efficacy portion) designed to generate the data needed to achieve FDA approval or authorization for use of one of the vaccine candidates. This is a randomized, placebo-controlled, observer-blind, dose-finding and vaccine candidate-selection study in healthy adults. The study is evaluating the safety, tolerability, and immunogenicity of the COVID-19 mRNA vaccine candidates.” –REF
- The “pivotal efficacy portion” is that “the study would be converted to a single Phase 1/2/3 study” in a July 2020 US Military communication – REF [combining phase 1+2+3 is unprecedented]
- Anticipate 7600 participants for 4 different SARS-CoV-2 mRNA/LNP vaccine canditates each at 3 dose levels of 10, 30 & 100 μg and control in 3 age groups (18-55, 65-85 and 18-85 years of age) – “with the first 15 participants at each dose level of each vaccine candidate comprising a sentinel cohort” [placebo control group]. Some groups received only 1 dose, others received 2 doses.
- BNT162a1,
- BNT162b1
- BNT162b2 (this ends up as the chosen candidate to move into Phase 2/3 trials – July 27, 2020)
- BNT162c2
- Study comprises 3 stages to “efficiently work towards selection of final candidate/dose level” (REF)
- Stage 1: to identify preferred vaccine candidate(s), dose level(s), number of doses, and schedule of administration
- Stage 2: an expanded-cohort stage
- Stage 3: a final candidate/dose large-scale stage
April 2020 [late April] – The German regulatory agency Paul Ehrlich Institute (PEI) approves the start of the first clinical trial in BioNTech’s vaccine program with the goal of choosing the best vaccine candidate from the four available. – TIMELINE, late April, exact date unknown – REF
- “In Germany, BioNTech began a Phase 1/2 study (BNT162-01) in late April 2020″ [note “01”]
- German study: “BNT162-01 is a dose-escalation trial investigating the safety and immunogenicity of COVID-19 mRNA vaccine candidates in healthy adults. – REF
- The primary objective of the study is to describe the safety and tolerability profiles of prophylactic BNT162 vaccine candidates after a single dose (for saRNA) or two doses separated by 21 days (uRNA and modRNA candidates).
- The secondary objective of the study is to describe the immune response to the vaccine in healthy adults, as measured by a functional antibody assay, such as virus neutralization.” [not reinfection/challenge effectiveness!]
April 9, 2020 – Pfizer and BioNTech Announce Further Details on Collaboration to Accelerate Global COVID-19 Vaccine Development – READ
April 9, 2020 – BioSpace: Pfizer Advances BioNTech COVID-19 Program and Plans Clinical Trials for Potential Treatments- READ
March 17, 2020 – Pfizer & BioNTech announce vaccine collaboration – READ
- mid-January 2020 – BioNTech starts Project Lightspeed to develop a vaccine against this new virus, and several vaccine candidates and conducts initial non-clinical studies. – TIMELINE
February 24, 2020 – FOIA Doc: BioNTech meeting with the Chinese CDC to discuss a possible Special Review Procedure – PDF, ARCHIVE, CREDIT
February 6, 2020 – FOIA Doc: BioNTech obtained feedback from the German Paul Ehrlich Institite (PEI) on plans for rapid vaccine developlnent following a Scientific Advice Meeting. Based on the PEI feedback, BioNTech refinded the clinical program plan and prepared a detailed protocol for FIH clinical study (BNT162) – PDF, ARCHIVE, CREDIT
2019
September 24, 2019 – BioNTech announces commencement of their Initial Product Offering (IPO), to become a publicly listed company – READ
September 2019 – The Bill and Melinda Gates foundation invests $55 million into BioNTech – SOUCRE: Motley Fool: 4 Coronavirus Vaccine Stocks the Bill & Melinda Gates Foundation Is Betting On – READ
June 27, 2019 – [Former FDA director nominated by Trump] Scott Gottlieb Elected to Pfizer’s Board of Directors – READ He served as the 23rd Commissioner of the U.S. Food and Drug Administration (FDA) from 2017 to 2019.
2018
November 19, 2018 – Biospace News: Reversing Course, Pfizer to Raise the Price of 41 Drugs in January [2019] – READ [Vaccines are more profititable than drugs, they go through a different regulatory pathway and come out the other side with exempt of liability if they get assigned to the childhood schedule]
October 16, 2018 – BioNTech CEO Dr Ugur Sahin, predicts mRNA technology can be used for rapid vaccine development – READ
October 14, 2018 at 10th World Health Summit – WATCH
- Albert Bourla (Pfizer Inc.) a vetenery medical doctor as chief operating officer, soon to become CEO spoke at the opening ceremony – EXCERPT
July 20, 2018 – SEC.gov: RESEARCH COLLABORATION AND LICENSE AGREEMENT by and between PFIZER INC. and BIONTECH RNA PHARMACEUTICALS GmbH and BIONTECH AG – READ, ARCHIVE, CREDIT
- Sec 3 Payments to BioNTech from Pfizer: “Notwithstanding the foregoing, Pfizer’s achievement of one of the Development Events … set forth in Section 3.3 in response to a request by a Governmental Authority or Regulatory Authority to perform such Development Event due to pandemic flu emergency conditions shall not trigger the payment of any preceding Development Event.”
Janaury 4, 2018 – BioSpace News: BioNTech Secures USD $270 Million in Series A Financing – READ
- BioNTech “previously completed a seed round fundraising in 2008 concurrent with its founding. The Series A round was led by the Redmile Group and joined by Janus Henderson Investors, Invus, Fidelity Management & Research Company and several European family offices. The Struengmann Family Office, an existing investor in BioNTech, also participated in the capital raise.”
2017
November 13, 2017 – Washington Post: Trump’s pick to lower drug prices is a former pharma executive who raised them – Alex Azar former Eli Lilly executive chosen as Secretary for Health and Human Services (HHS) – READ, Fox News – READ, Following Tom Price resignation – READ
July 14, 2017 – BioNTech forms a “Patent Sublicense Agreement” with mRNA RiboTherapeutics, Inc – the company that owns the mRNA pseudouradine components. – READ, CONTEXT
March 10, 2017 – Washington Post: Trump to select Scott Gottlieb, a physician with deep drug-industry ties, to run the FDA – READ, CNN – READ, Allegedly endorsed by Pfizer – SOURCE
- Gottlieb in June 2019 elected to Pfizer’s board – READ
February 17, 2017 – USA Today: Corporations gave millions to Donald Trump inauguration – [$100 million total] – READ, CREDIT
- “Pfizer, Dow Chemical and Bank of America gave $1 million apiece to the inaugural committee …celebrating Trump’s Jan. 20 swearing-in”. Pfizer’s donation was made on Dec. 21, 2016.
- “Shortly before he took office, Trump said pharmaceutical companies were “getting away with murder”…In a Jan. 31 [2017] meeting with pharmaceutical executives at the White House, Trump was less critical…”
2016
August 1, 2016 – Pfizer: Pfizer aims to become industry leader in Gene Therapy with Aquisiton of Bamboo Therapeutics Inc – READ, ARCHIVE, Pfizer gene therapy – WEB
- Jude Samulski, Chief Scientific Officer and Executive Chairman of Bamboo Therapeuitics and a leading expert in the field of rAAV vectors with more than 25 years of experience, will be joining Pfizer. Dr. Samulski, together with the Bamboo team, will play a key role in helping to develop and accelerate Pfizer’s capabilities in gene therapy.”
- Dr R. Jude Samulski is the cofounder of AskBio (2003) and founder of AAV delivery technology, out of UNC Chapel Hill. Also “Dr. Samulski is a former member of the Recombinant DNA Advisory Committee (RAC), a committee tasked with assisting the FDA with approving or disapproving gene therapy clinical trials in the United States. Dr. Samulski also frequently serves as a gene therapy consultant to the FDA. In 2008, Dr. Samulski was recognized by the American Society of Cell and Gene Therapy with the Inaugural Lifetime Achievement Award for his work.” – REF
- “Pfizer also entered into a collaboration and license agreement with Emeryville, Calif.-based 4D Molecular Therapeutics (4DMT) to discover and develop targeted next-generation rAAV vectors for cardiac disease.” – REF
March 22, 2016 – PRESS RELEASE: Pfizer Joins The Human Vaccines Project To Help Decode The Immune System – ARCHIVE, SOURCE, (and accelerate new vaccine technologies) – TIMELINE
2014
2014 – “In 2014, Pfizer established within the company’s Rare Disease Research Unit the Genetic Medicines Institute (GMI) in London, UK, which is a dedicated gene therapy research group under the direction of leading gene therapy researcher Michael Linden”. –REF
2010
April 7, 2010 – Corporate Watch: Pfizer “Too Big To Nail” – The cost of doing business! – WATCH, ARCHIVE
Pfizer and the Feds cut a deal and this is how they did it -> WATCH
“This activity will continue...”
Drew Griffin – Reporter
2002
April 16, 2002 – US Patent US 6372224B1 is awarded to Pfizer Inc. titled “Canine coronavirus S gene and uses therefor” (submitted January 28, 2000) – READ, PDF
- [Spike sequence of Canine Coronavirus (CCV) is useful] for use in vaccine and therapeutic agents.
1984
1984 – Pfizer’s Medical & Academic Partnerships funding program begins (Private-Public partnership influence) – READ
- In partnership with CDC, NIH, AMA, American Public Health Association and more – ARCHIVE
- Pfizer Public Heath – launched ~2002 – ARCHIVE
- 2003 – Through the CDC Foundation Fellowships – “Encourages physicians to pursue epidemiology through an innovative training program that introduces physicians-in-training to public health and the field practice of epidemiology” – ARCHIVE
- From 2004 sponsoring Association of Schools of Public Health – ARCHIVE, ARCHIVE2
- 2003 – Advancing Healthy Populations: Pfizer Guide to Careers in Public Health – This guide inspires pursuit of work in public health and provides a practical resource for making the most of potential opportunities across a broad range of career choices. – ARCHIVE, PDF
- “Immunization” is mentioned 23 times!