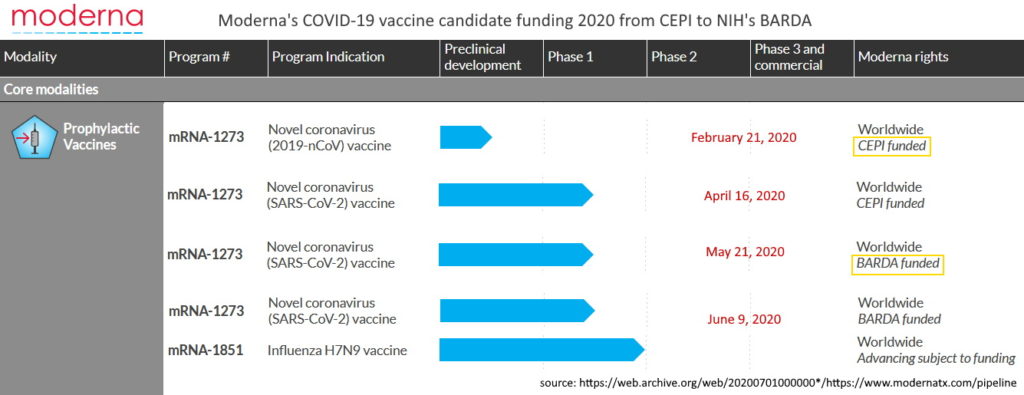
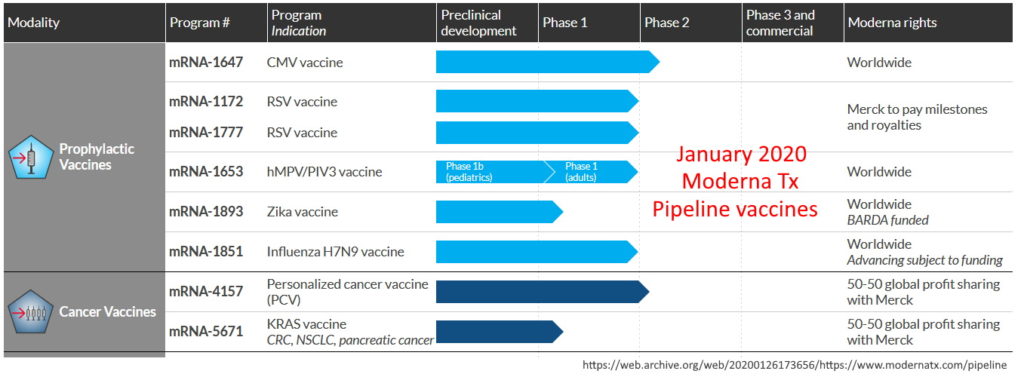
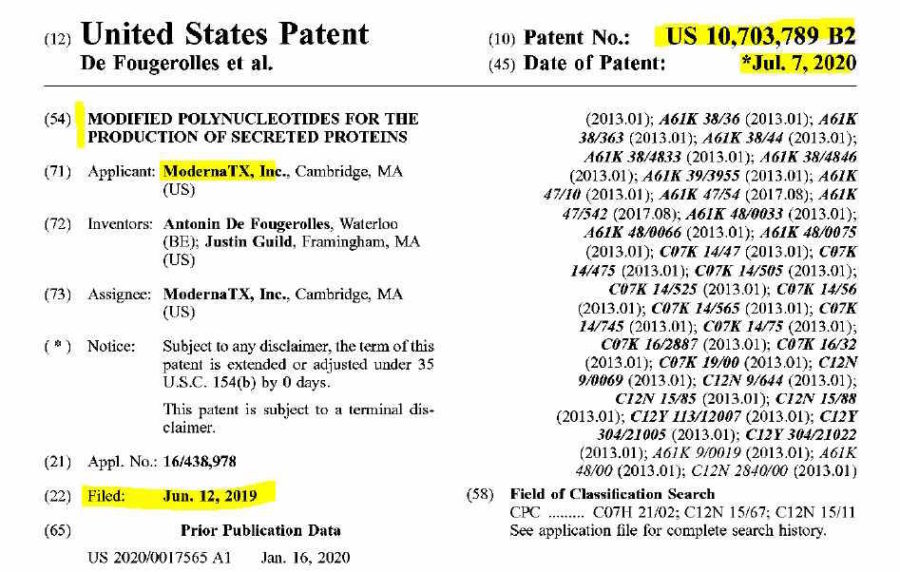
Moderna, based in Cambridge, Massachusett., began it’s inception in 2010 following Derek Rossi’s breakthrough paper, demonstrating chemically modified mRNA could reprogram adult human fibroblast cells – the prelude to gene therapy. The main investor in the startup was Flagship Pioneering.
The National Institute for Allergy and Infectious Diseases has partnered with Moderna on the development of their vaccine. Scientists at NIAID made the vaccine’s construct, or prototype, and the agency ran the Phase 1 trial. [see May 18, 2020]
As of January 2020 Moderna had NOT yet brought a ANY product to market, let alone a “vaccine”, though it had “a variety of vaccines for infectious diseases in its pipeline.” Moderna product pipeline – ARCHIVE
About Moderna
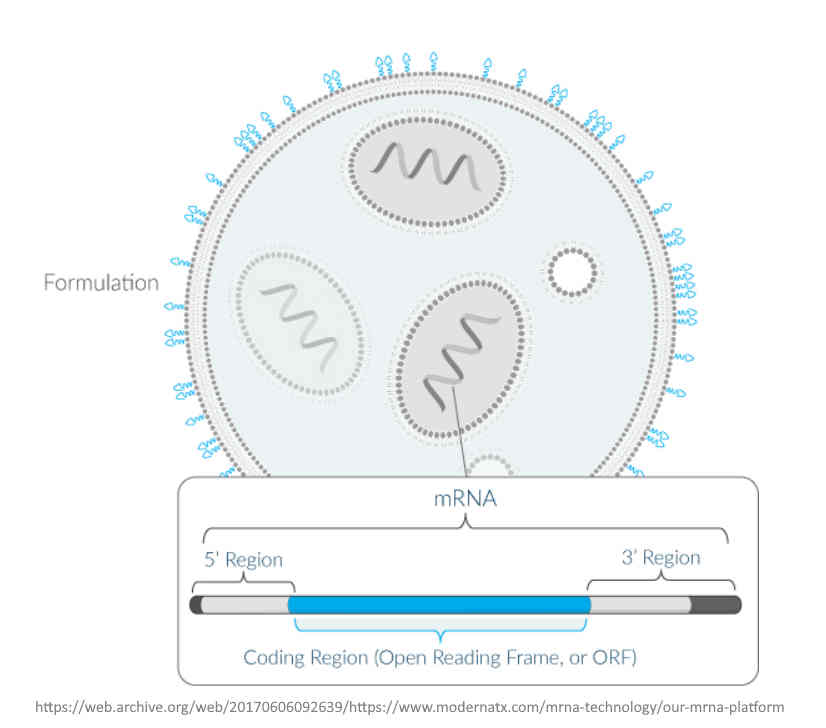
Moderna (Modified RNA) is an “mRNA technology company designed to maximize the promise of mRNA science.” – ARCHIVE
An “Operating System“, the “Software of Life” – Moderna “set out to create an mRNA technology platform that functions very much like an operating system on a computer. It is designed so that it can plug and play interchangeably with different programs. In our case, the “program” or “app” is our mRNA drug – the unique mRNA sequence that codes for a protein.” – Moderna ARCHIVE1, ARCHIVE2

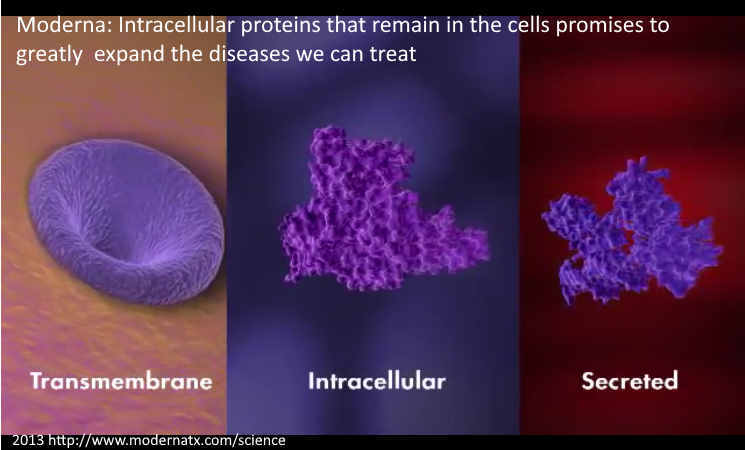
“We have strategic alliances with Merck on select commercial vaccines, and BARDA and DARPA on global health vaccine programs.” REF [Merck are the original patent holders of ivermectin!]
SPIKEVAX is the trade name of Moderna’s mRNA PLATFORM, no matter what protein it encodes – READ
Clinical trails
links to ClinicalTrial.gov [to be updated]
- February 25, 2020 – ClinicalTrials.gov identifier NCT04283461: Safety and Immunogenicity Study of 2019-nCoV Vaccine (mRNA-1273) for Prophylaxis of SARS-CoV-2 Infection (COVID-19) – Sponsor: NIAID, Collaborator: Moderna – ARCHIVE, READ
- July 14, 2020 – ClinicalTrials.gov Identifier NCT04470427: A Study to Evaluate Efficacy, Safety, and Immunogenicity of mRNA-1273 Vaccine in Adults Aged 18 Years and Older to Prevent COVID-19 ( Moderna, BARDA, NIAID) Phase 3 COVE clinical trial – READ, ARCHIVE, PRESS
- December 2, 2020 – ClinicalTrials.gov identifier NCT04649151: A Study to Evaluate the Safety, Reactogenicity, and Effectiveness of mRNA-1273 Vaccine in Adolescents 12 to <18 Years Old to Prevent COVID-19 (TeenCove) – (Moderna + BARDA) – READ, ARCHIVE, PRESS
- January 21, 2021 -ClinicalTrials.gov identifier NCT04677660: A [Phase 1/2] Study of TAK-919 in Healthy Japanese Adults (COVID-19) [Japan] – READ, PRESS
Moderna building “mRNA platform” manufacturing facilities
Beginning August 2021 Moderna begun signing “deals “long-term strategic partnerships” with the governments of Australia, UK, Canada to build manufacturing facilities in each country for their brand new “mRNA platform” technology products – pending regulatory apporval of course! No double they will cover any potential infectious disease, based on their “experience” with COVID-19
“The uniquely challenging year of 2020 for all of society proved to be an extraordinary proof-of-concept period for Moderna…we are extending our mRNA development work to a total of 24 programs across five therapeutic areas”
Stéphane Bancel, Moderna CEO, January 11, 2021

Articles in reverse chronological order
Many links are Moderna’s own PRESS RELEASEs, which allows the timeline of events to be captured.
It’s interesting looking back!
Moderna Press Releases – HERE
Moderna Blog – ARCHIVE
2025 Moderna Australia – WEBSITE
FDA – SPIKEVAX and MNEXSPIKE
2025
July 10, 2025 – PRESS RELEASE: Moderna Receives Full U.S. FDA Approval for COVID-19 Vaccine, SPIKEVAX, in Children Aged 6 Months Through 11 Years at Increased Risk for COVID-19 Disease – READ, READ, ARCHIVE, LABEL, Gateway Pundit :…Despite Mounting Safety Concerns and Public Distrust – CREDIT, FDA’s decision – PFD
May 30, 2025 – FDA Letter to Moderna – BLA for BLA for COVID-19 Vaccine, mRNA (MNEXSPIKE) – PDF, ARCHIVE
- The review of this product was associated with the following National Clinical Trial (NCT) numbers: NCT04813796, NCT05137236, NCT05815498, NCT04927065, and NCT04649151
May 28, 2025 – AP: Trump administration cancels $766 million Moderna contract to fight pandemic flu – READ, Reuters – READ, Just the News – READ, VC Post – READ, CREDIT
- “Moderna in January [2025] was awarded $590 million by the Biden administration to advance the development of its bird flu vaccine, and support the expansion of clinical studies for up to five additional subtypes of pandemic influenza.
- This was in addition to $176 million awarded by the U.S. Department of Health and Human Services (HHS) last year to complete the late-stage development and testing of a pre-pandemic mRNA-based vaccine against the H5N1 avian influenza.”
May 28, 2025 – Moderna PRESS – Moderna Announces Update on Investigational Pandemic Influenza Program – READ
2024
December 5, 2024 – Victorian Government: New Moderna mRNA facility opens in AUSTRALIA – READ, READ
- Moderna’s new, world-class vaccine manufacturing facility is officially open, giving Victorians access to locally manufactured mRNA vaccines and medicines. – The facility is expected to produce its first mRNA vaccines in 2025.
- “With Moderna and BioNTech establishing major hubs in the state, Victoria is the only place in the world where both mRNA leaders host research and development (R&D) and manufacturing operations, as well as the first commercial scale, end-to-end mRNA vaccine facility in the southern hemisphere.”
- Population scale mRNA manufacturing – with a capacity to produce up to 100 million vaccine doses per year – READ
- ‘This facility gives Australia sovereign capability to produce its own mRNA vaccines in response to a future pandemic“
December 4, 2024 – Moderna Australia Press Release: MODERNA OPENS FIRST & ONLY COMMERCIAL-SCALE MRNA MANUFACTURING FACILITY IN THE SOUTHERN HEMISPHERE – PDF
September 12, 2024 – Axios: Moderna scales back vaccine ambitions as COVID shot revenue plunges – READ, Gateway Pundit – CREDIT
- “Moderna’s technology is promising, but COVID vaccines are no longer enough to sustain the full scale of its R&D ambitions.”
- Aug 1, 2024 – Moderna Q2 2024 report – READ
June 7, 2024 – Moderna Press: Moderna Files FDA Application for the JN.1 Targeting COVID-19 Vaccine – to be Moderna’s Spikevax 2024-2025 formula, targeting the SARS-CoV-2 variant JN.1 to be available August 2024 – READ
- “The submission is based on guidance from the U.S. FDA, which advised that COVID-19 vaccines should be updated to a monovalent JN.1 composition for the 2024-2025 season. This guidance aligns with the [April 26, 2024] recommendations from the World Health Organization (WHO) Technical Advisory Group on COVID-19 Vaccine Composition (TAG-CO-VAC) and the European Medicines Agency’s Emergency Task Force (ETF) on the use of a monovalent JN.1 lineage for COVID-19 vaccine antigen composition.”
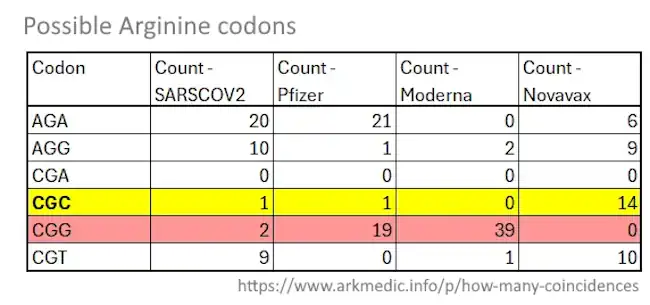
May 19, 2024 – Arkmedic Substack: How many coincidences…before it becomes mathematically impossible? A deep dive into the possibility that similarities in the design of the genetic COVID vaccines that magically appeared overnight in February 2020 were more than coincidental. – READ
- “Now, in case you are already lost “#CoptiGate” was all about the fact that the Pfizer and Moderna vaccines completely replicated the original COVID virus spike protein to the exact amino acid of the 1273 amino acid sequence that made up that protein, but changed the underlying nucleotide sequence that provided the code for that amino acid sequence.”
- Moderna converted nearly all its Arginines codons to CGG instead of the more optimised CGC, with 93 CGG “triplets” (i.e. sequences that just exist in the sequence) – why does maximising the CGG sequence matter?
- “…all these approaches have the impact of dropping PAM sequences into the code under cover of “codon optimisation” with plausible deniability against the idea that dropping PAM sequences into the code was what they were actually trying to do.” – CRISPR needs a PAM! – READ
A PAM sequence is a triplet of CGG (preferred), or any other nGG triplet – irrespective of whether it is in a coding location – that enables an anchor for CRISPR-Cas9 gene editing
May 4, 2024 – Gateway Pundit: Moderna Posts $1.2 Billion Loss as Demand For COVID Jab Collapses, Promises Investors ‘Next-Generation’ Vaccine to Tackle New Strains – READ
- Epoch Times: Moderna Posts $1.2 Billion Loss as Sales of COVID-19 Vaccine Plunge 94 Percent – READ
May 2, 2024 – Moderna Press Release: Moderna Reports First Quarter 2024 Financial Results and Provides Business Updates – READ
May 1, 2024 – Gateway Pundit: CDC Concealed Evidence Linking Deaths to Pfizer and Moderna COVID-19 Vaccines, Internal Documents Reveal – READ, Epoch Times – SOURCE, TWEET
- “The internal file obtained by The Epoch Times details numerous cases of individuals who died following COVID-19 vaccination, with autopsy reports attributing causes of death directly to vaccine-related complications such as myocarditis and pericarditis…”
- “Despite these [myocarditis] findings, the CDC maintained that it had seen no signs linking the Moderna and Pfizer mRNA vaccines to any deaths reported to the Vaccine Adverse Event Reporting System (VAERS).”
April 24, 2024 – Modern Press Release: Moderna and OpenAI Collaborate To Advance mRNA Medicine – READ
March 27, 2024 – Moderna Press Release: Moderna Advances Multiple Vaccine Programs to Late-Stage Clinical Trials – READ
- Phase 3 – combination vaccine against flu and COVID-19,
- Phase 3 Cytomegalovirus (CMV)
- Epstein-Barr virus (EBV)
- Herpes simplex virus (HSV)
- Varicella-Zoster virus (VZV)
March 26, 2024 – Moderna Press Release: Moderna Achieves Positive Interim Results from Phase 3 Trial of Next-Generation COVID-19 Vaccine – READ
- mRNA-1283, Moderna’s’s next-generation COVID-19 vaccine, induced a more robust immune response compared to Spikevax® COVID-19 vaccine, mRNA-1273.222. mRNA-1283 met the primary endpoints of its Phase 3 NextCOVE (NCT05815498) clinical trial, where mRNA-1283 was shown to elicit a higher immune response against both the Omicron BA.4/BA.5 and original virus strains of SARS-CoV-2, compared to mRNA-1273.222!
- Next-generation mRNA vaccine design offers the potential of longer shelf life and storage advantages, and paves the way for combination vaccine against influenza and COVID-19, mRNA-1083
- mRNA-1283 is a critical component of our combination vaccine against flu and COVID-19 (mRNA-1083), and this milestone gives us confidence in our ability to bring this much needed vaccine to market.”
2023
November 14, 2023 – Daily Clout: Report 91: FDA Based Moderna’s mRNA COVID Vaccine Approval on Test of a Completely Different Non-COVID Vaccine. Only Males Included in Test – READ
Novmeber 17, 2023 – Alex Berenson – EXCLUSIVE: Moderna has halted a trial of a new mRNA vaccine for young people after a suspected myocarditis case – Phase 1 trail for Moderna’s Epstein-Barr virus vaccine! – READ
October 30, 2023 – CEPI and Moderna Harness mRNA Technology to Advance 100 Days Mission – rEAD
October 3, 2023 – Unacceptable Jessica Substack: Remember that 4chan post from 2020? – Is it science fiction? Is it real? – READ, Alleged anonymous Moderna engineer insider from Sep 2020
August 15, 2023 – BioTech Dispatch: A major day for Moderna as the company builds its Australian presence – READ
- Dr Craig Rayner, the director of Moderna’s Regional Centre for Respiratory Medicines and Tropical Diseases, said, “Moderna is proud to invest in the advancement of Australia’s mRNA scientific capabilities…”
August 3, 2023 – Australian Senate Public Hearings: COVID-19 Vaccination Status (Prevention of Discrimination) Bill 2022 and the Fair Work Amendment (Prohibition COVID-19 Vaccine Discrimination) Bill 2023 – WATCH, TWEET, Sen Hanson – EXCERPT, Sen Antic (Q 4 Moderna)- EXCERPT
Australian Senators vs Moderna– Video excerpts – HERE , vs TGA – HERE (credit Penny Butler)
July 26, 2023 – Epoch Times: Subclinical Heart Damage More Prevalent Than Thought After Moderna Vaccination: Study – “Damage to the heart is more common than thought after receipt of Moderna’s COVID-19 booster” – READ, CREDIT
June 19, 2023 – Epoch Times: Australia Removes Moderna Vaccine for Children Under 5 – READ, Dept Health – ARCHIVE
- ATAGI noted “clinical trial of 5,500 children aged six months up to five years demonstrated that the Moderna COVID-19 vaccine provided only modest protection against infection, while safety data reported patterns of vaccine-related adverse events.” for this low risk group! “…the side effect profile for this vaccination needs to be considered in the risk-benefit discussion”- REF
- “Several deaths attributed to the vaccine have been reported to TGA Database of Adverse Event Notifications (DAEN) for children aged 7 and 9 – This info came to light ONLY following Freedom of Information request by an Australian doctor”
- Moderna’s paediatric formulation of the Moderna COVID-19 vaccine (Spikevax) which was approved July 2022 is now no longer available in Australia
- In February 8, 2023 ATAGI no longer recommended boosters for children under 18 years – TIMELINE, IMAGE
“A third independent medical party should examine the evidence as the TGA has a conflict of interest because they approved the vaccines and would therefore be held responsible for the deaths of these children due to poor regulatory oversight,”
Senator Gerard Rennick told The Epoch Times
April 24, 2023 – TGA: Moderna’s COVID-19 vaccine SPIKEVAX receives approval for full registration – READ, ARCHIVE, CREDIT
- This is the first COVID-19 vaccine to receive full registration in Australia
- Clinical trials had 15.1 adverse events/1,000 doses – which doesn’t seem to alarm TGA!
- There are currently 7,442 reported adverse events in DAEN, 36 deaths, This is Australia’s equivalent to VAERS
April 22, 2023 – Alex Berenson Sugstack: Unreported Truths: URGENT: Moderna hid serious side effects suffered by its Covid vaccine recipients when it reported clinical trial results for the shot – Moderna scientists said in a 2021 paper no mRNA jab recipients in the trial had “serious adverse effects.” In fact, 14 ultimately did, including three miscarriages. No placebo recipients did – READ
January 26, 2023 – FDA | VRBPAC meeting to Discuss Future Vaccination Regimens Addressing COVID-19 – Moderna COVID-19 Bivalent Vaccines Primary Series and Booster – presentation by Moderna – SLIDE, All meeting material – HERE, WATCH
January 25, 2023 – The Highwire | Tracy Beanz & Michelle Edwards (from UnCoverDC): mRNA is Not Going Away: Moderna CEO has Permanent Global Plans – and DARPA – READ, WATCH
- At WEF in Davos, Moderna CEO Stéphane Bancel declared he would “like to have mRNA capacity on every continent.” No surprise there as Moderna has been “developing mRNA gene-editing technology for the Defense Advanced Research Projects Agency (DARPA) for over a decade.”
January 20, 2023 – TGA: TGA commences evaluation of Moderna COVID-19 vaccine (SPIKEVAX) for potential transition to full registration – READ, TIMELINE
- Spikevax = the mRNA vaccine platform, not the protein with which the mRNA codes!
January 18, 2023 – World Economic Forum – State of the Pandemic with Stéphane Bancel (Moderna CEO) etc – WATCH
January 18, 2023 – Reuters: Davos 2023: Moderna CEO in talks with China to supply COVID vaccines – READ
January 12, 2023 – NY Post: Moderna under fire for 400% price hike of COVID vaccine: ‘Outrageous’ – READ
- Moderna “could raise the price of the vaccine from its current $26 per dose to between $110 and $130 per shot, according to The Wall Street Journal.” – READ
January 10, 2023 – Dept Health Australia published and update stating “The Moderna (original) vaccine [forumlation] is no longer being manufactured by Moderna, therefore no more Moderna (original) vaccine is being imported and the stock supply within Australia is expected to expire in early 2023″ – ARCHIVE, READ
January 9, 2023 – Stat News: Moderna plans to follow in Pfizer’s footsteps, charge up to $130 for Covid-19 vaccine in U.S. – as disclosed to Wall Street Journal – READ, ARCHIVE, Aaron Siri – CREDIT
2022
December 27, 2022 – Dr John Campbell: Massive UK Moderna partnership – WATCH – Moderna expands, UK, Canada and Australia plants – for a relatively untested technology product!- EXCERPT
December 22, 2022 – UK Government PRESS RELEASE: UK cements 10-year-partnership with Moderna in major boost for vaccines and research – Moderna to invest in mRNA research and development (R&D) in the UK, and build a state-of-the-art vaccine manufacturing centre with the ability to produce up to 250 million vaccines a year. – READ
December 21, 2022 – PRESS RELEASE: Moderna Finalizes Strategic Partnership with UK Government – READ, following on from June 2022 release – READ,
November 27, 2022 – Epoch Times via Zero Hedge: Another Study Finds Heart Inflammation Higher After Moderna Vaccination Versus Pfizer – READ, Canadian Medical Ass. Journal – STUDY
November 21, 2022 – Canadian Med Ass J: Observed versus expected rates of myocarditis after SARS-CoV-2 vaccination: a population-based cohort study – Naveed et al – READ
December 15, 2022 – Monash University Aust.: Moderna appoints Dr Craig Rayner as Director, Regional Centre for Respiratory Medicines and Tropical Disease – READ, READ
- No mention of Rayner history with Australia’s Dept of Defence Medical Countermeasures! – TIMELINE
- June 4, 2020 – A report to the Defence Department three years ago with lead author, Dr Craig Rayner, warned that Australia lacks the facilities and know-how to make its own pandemic vaccines. “Australia imports over 90 per cent of medicines and is at the end of a very long global supply chain, making the nation vulnerable to supply chain disruptions,” the report said. – REF
December 13, 2022 – Judicial Watch: Judicial Watch: FDA Records Show Significant Number of mRNA Test Rats Born with Skeletal Deformations – Moderna mRNA vaccine, FOIA documents indicate a “statistically significant” number of rats were born with skeletal deformations after their pregnant mothers were injected with the vaccine.” – READ
November 14, 2022 – Daily Mail: Pfizer and Moderna launch trials to track whether health issues arise YEARS after getting their Covid vaccines – READ, but they got rid of the trial control group Jan 2021 – TIMELINE
November 7, 2022 – Global News Canada: Feds hold groundbreaking ceremony for Moderna’s mRNA vaccine factory in Montreal area – READ
November 3, 2022 – Moderna PRESS RELEASE: Moderna Reports Third Quarter 2022 Financial Results and Provides Business Updates – READ
- Third quarter 2022 revenues of $3.4 billion
- 2022 expected to be $18 to $19 billion
- Phase 3 RSV vaccine efficacy data could read out this winter [note: increased incidence in RSV is a side effect of the CV19 vaccine in children – REF]
- Phase 3 flu vaccine immunogenicity data expected in 1Q 2023
October 20, 2022 – Igor Chudov Substack: CDC Data: Moderna Causes 42% MORE Miscarriages Compared to Pfizer – CDC Presentation Provides Irrefutable Proof – READ
October 19, 2022 – NEJM: Evaluation of mRNA-1273 Vaccine in Children 6 Months to 5 Years of Age by Anderson et al (KidCOVID Study Group) – READ
- “Curious why this isn’t getting more attention” 1 in 200 toddlers had serious adverse event but still safe in children!!! – Dr Tenpenny – GETTR
October 16, 2022 – DARPA and Moderna pioneered the idea behind mRNA vaccines – DARPA has openly bragged on Twitter that Moderna’s mRNA vaccine technology, was a product of their ADEPT program by Rhoda Wilson – READ
- The paper trail shows “Moderna is just another front in the Biodefence Mafia”
- The “cheerleaders of this technology…are all looking for technology that is easy, rapid, and cost-effective for development and manufacture.”
October 12, 2022 – Moderna PRESS RELEASE: Moderna Receives FDA Authorization for Emergency Use of Omicron-Targeting Bivalent COVID-19 Booster Vaccine for Children and Adolescents 6 to 17 Years of Age – READ
October 9, 2022 – Sky News: Moderna to release updated booster shot – WATCH, EXCERPT, Gateway Pundit – ARTICLE
Bancel: “We are now in a super exciting program where we inject mRNA in people’s heart after a heart attack to grow back new blood vessels and re-vascularize the heart”
Reporter: “The irony of COVID is that is really has in some ways allowed you to go and develop these other areas because of the revenues that came through the door.”
Bancel: “You’re 100% right”
EXCERPT
October 6, 2022 – NEJM: A Bivalent Omicron-Containing Booster Vaccine against Covid-19 by Chalkais et al (Moderna) – READ, Daily Clout – CONTEXT
- This paper was available to the FDA advisory commettee “at the time of the committee vote demonstrated that more people became infected with the bivalent boosters (3.2%) than the people who received the original booster shot (1.9%).” – REF
September 22, 2022 – Vaccine: Serious adverse events of special interest following mRNA COVID-19 vaccination in randomized trials in adults – Fraiman, Doshi et al – READ
- “Secondary analysis of serious adverse events reported in the placebo-controlled, phase III randomized clinical trials of Pfizer and Moderna mRNA COVID-19 vaccines in adults (NCT04368728 and NCT04470427), focusing analysis on Brighton Collaboration adverse events of special interest.”
- The reanalysis of Moderna clinical trial data revealed a serious adverse event rate of 15.1 per 10,000 doses – REF
September 21, 2022 – FiercePharma: Moderna’s new booster launch tripped up by production issues at Catalent plant – “unexplained supply problems” – READ, Hedley Rees Substack: FDA unearths a minefield of quality issues at the contractor manufacturing Moderna’s jabs – ARTICLE, FDA inspection – FORM 483 – HERE
September 9, 2022 – EU Parliament: Romanian MEP Cristian Terheș Takes Moderna and AstraZeneca to Town With an Onslaught of Questions – WATCH
August 31, 2022 – Moderna PRESS RELEASE: Moderna Receives FDA Authorization for Emergency Use of Omicron-Targeting Bivalent COVID-19 Booster Vaccine for Adults 18 Years and Older – READ
August 30, 202 – Moderna PRESS RELEASE” Therapeutic Goods Administration Provisionally Approves Moderna’s Omicron-Containing Bivalent Booster Candidate, MRNA-1273.214, For Australia – “Australia becomes among the first countries in the world to approve the use of a next-generation bivalent COVID-19 vaccine” – READ
August 29, 2022 – Australia’s Therapeutic Goods Administration (TGA) provisionally approved Moderna’s bivalent COVID-19 vaccine, elasomeran/imelasomeran (SPIKEVAX Bivalent Original/Omicron) for use as a booster dose in adults 18 years and over. This is the first bivalent COVID-19 vaccine approved for use in Australia. – READ, ARCHIVE
- All COVID-19 vaccine provisional approvals – HERE, ARCHIVE
All “provisionally approved” products are part of Black Triangle Scheme for minimum of 5 years – READ
August 26, 2022 – Reuters: Moderna sues Pfizer/BioNTech for patent infringement over COVID vaccine – READ, NPR – READ
August 26, 2022 – Moderna PRESS RELEASE: Moderna Sues Pfizer and BioNTech for Infringing Patents Central to Moderna’s Innovative mRNA Technology Platform – READ
August 26, 2022 – Epoch Times: Moderna Vaccine Trials Contained ‘Mostly Irrelevant Studies’ and ‘Deceptive Practices’: Veteran Pharma Analyst – READ
- Moderna’s internal documents regarding their COVID vaccine trials, obtained via a Freedom of Information Act request by Judicial Watch, show that most of their studies submitted for approval to the FDA were “irrelevant” and did not follow Good Laboratory Practices (GLP), according to a former pharma executive.
- 400 of the 700 pages of the BLA package were on non-Spikevax products i.e. mRNA-1653 and not mRNA-1273 (Spikevax)
- “Ignore the cargo, focus on the vehicle”
- “Moderna claimed that the active substance mRNAs of SPIKEVAX does not need to be studied for toxicity and can be replaced with any other mRNA without further testing,” she said.
- “The FDA did not push back on this preposterous claim. The company is claiming that the active drug substance of a novel medicine does not need to be tested for toxicity.”
- The documents have not been made public, but were analyzed by former pharma executive Alexandra Latypova and reviewed by The Epoch Times.
August 17, 2022 – Unlimited Hangout: RNA for Moderna’s Omicron Booster Manufactured by CIA-Linked Company – Since late last year, messenger RNA for Moderna’s COVID-19 vaccines, including its recently reformulated Omicron booster, has been exclusively manufactured by a little known company with significant ties to US intelligence – called National Resiliance – – READ, Expose News – READ, CHD – – READ, Dr Huff – govt moving mRNA out of NIH – GETTR
- “Last September [2021], it was quietly announced that a company called National Resilience (often referred to simply as Resilience) would begin manufacturing the mRNA for Moderna COVID-19 vaccine products.”
August 15, 2022 – Daily Clout: Moderna CEO Stéphane Bancel Compares COVID Shot to iPhone, Saying People Will Receive New Version Each Year – READ, WATCH
August 15, 2022 – Monash University Melbourne: Moderna to build manufacturing facility at Monash – Australia – READ, Aust Govt – READ, ARCHIVE,
- To be built at Monash Technology Precinct, Melbourne, under agreement with Victorian government, an agreement started Dec 13, 2021.
- The Australian Government, Moderna and the Victorian Government have finalised partnership arrangements to establish an mRNA vaccine manufacturing facility at Monash University in Melbourne. – READ
August 15, 2022 – GB News: Britain becomes first country in world to approve Moderna’s new Covid jab that targets Omicron variant – BiValent Vaccine – READ, TIMELINE
August 9, 2022 – CHD | The Defender: Moderna Clinical Trials Terribly Flawed — and FDA Knew It, Former Pharma Executive Tells RFK, Jr. – READ & WATCH
July 19, 2022 – AUSTRALIA: “A paediatric formulation of the Moderna COVID-19 vaccine (Spikevax) was provisionally approved by the Therapeutic Goods Administration (TGA) on 19 July 2022 for use in children aged 6 months to 5 years” – REF, On Aug 3, 2022 ATAGI recommended the shot for this age group
July 18, 2022 – Trial Site News : Moderna FOIA Bombshell Reveals Alarming Problems with FDA Approval of SPIKEVAX with Alexandra Latypova (“Sasha”) – WATCH
- Sasha has gone through around 700 pages of Judical Watch’s Moderna FOIA documents that are yet to be released to the public. They assessed other unrelated mRNA’s not spike protein coding mRNA in their application!
July 18, 2022 – Moderna’s new variant-busting COVID vaccine starting trial in Israel – An Israeli hospital announced it is part of a trial for Moderna’s new variant-busting coronavirus vaccine. – READ
July 18, 2022 – Moderna PRESS RELEASE: [Australia] Therapeutic Goods Administration Grants Provisional Approval for Moderna’s COVID-19 Vaccine in Children Aged Six Months to Five Years – READ
July 14, 2022 – Canada approves Moderna vaccine for children as young as SIX MONTHS old – The vaccine is the first approved in Canada for children under 5. – READ, Health Canada – HERE
- French health authorities last year advised against Moderna for those under 30, due to “comparatively higher risks of heart-related problems,”
July 14, 2022 – Epoch Times: Top Regulator: ‘Severe Allergic Reaction’ a Side Effect of New COVID-19 Vaccine – READ
July 7, 2022 – Trial Site News by Sasha Latypova: Moderna’s Non-clinical Summary for Spikevax – Evidence of Scientific and Regulatory Fraud – RE Judicial Watch FOIA documents from FDA via HHS – READ
- Analysis of 699 pages of Moderna documents used by FDA for vaccine EUA, gained from Judicial Watch FOIA
- The FDA “accepted fraudulent test designs, substitutions of test articles, glaring omissions and whitewashing of serious signs of health damage by the product, then lied to the public on behalf of the manufacturers.”
- “Moderna’s documents are poorly and often incompetently written — with numerous hypothetical statements unsupported by any data, proposed theories, and admission of using unvalidated assays and repetitive paragraphs throughout,”
- CHD: FDA Colluded With Moderna to Bypass COVID Vaccine Safety Standards, Documents Reveal – ARTICLE
- NIH documents submitted – conflict of interest
- “The FDA and Moderna lied about reproductive toxicology studies in public disclosures and product labeling.”
June 30, 2022 – Trial Site News: Pharmaceutical Expert Says FDA Colluding with Pfizer and Moderna, Not Addressing Alarming Data with Alexandra Latypova (“Sasha”) – WATCH
June 29, 2022 – We The People USA: 700 Million Worldwide Will Die From CV19 VAX By 2028: Dr. David Martin provides insight into Moderna – WATCH
June 23, 2022 – The Dossier Substack: Already Expired: Moderna’s upcoming Omicron shot is formulated for a variant that no longer exists – BA.1 is no longer spreading – READ
June 22, 2022 – UK Government PRESS RELEASE: Moderna to open vaccine research and manufacturing centre in UK – NHS patients to have access to next generation of mRNA vaccines and treatments – READ
June 22, 2022 – CNBC: Moderna to build new vaccine facility in Britain – READ
- Production expected to start 2025, and UK govt (tax payer) committed to purchase for next 10 years (for a relatively untested mRNA technology product!)
- Priority production for booster “COVID, flu and Respiratory Syncytial Virus (RSV)”.
- Moderna has announced manufacturing facilities in Kenya, Canada and Australia.
June 17, 2022 – Moderna PRESS RELEASE: Moderna Receives FDA Authorization for Emergency Use of Its COVID-19 Vaccine for Children 6 Months of Age and Older – READ, Moderna press release – READ
June 11, 2022 – Team Enigma: Moderna and Pfizer: Reproductive Toxicology Studies from FOIA Documents – WATCH
June 8, 2022 – MODERNA PRESS RELEASE: Moderna Announces Omicron-Containing Bivalent Booster Candidate mRNA-1273.214 Demonstrates Superior Antibody Response Against Omicron – READ
May 24, 2022 – Forbes: Billionaire Moderna CEO Stéphane Bancel Pledges Stock Options Worth $355 Million To Undisclosed Charitable Causes – READ, he became a billionaire for the first time in April 2020! – READ
May 17, 2022 – PRESS RELEASE: Moderna Announces Advancements in mRNA Platform Science for Application Across Multiple Diseases at [Moderna’s 5th annual] Science and Technology Day [for analysts and investors] – “mRNA is inherently unstable” – READ, READ2
- “Moderna continues to highlight investments in the expansion of the utility of the mRNA platform, characterization of the Company’s vaccines, biodistribution of vaccines, and using clinical data to predict vaccine dosing”
- “…the effective delivery of mRNA-based medicines is enabled by encapsulating the mRNA in tiny lipid (fat) droplets, known as lipid nanoparticles (LNPs) in order to protect it against degradation and facilitate uptake by cells. Moderna announces advances in developing numerous proprietary LNPs, each suited to target different cell types and optimized for different routes of administration”
- “the Company has invested in designing mRNA delivery systems tailored to targeting pulmonary disease” via inhalation [future nasal vaccines??] “new LNP formulation that addresses many of the challenges associated with lung delivery of mRNA.” Moderna is collaborating with Vertex [1, 2]
May 17, 2022 – Team Enigma: Moderna – Who is Behind this House of Cards? Sasha found evidence of fraud, corruption of science, and financial machinations. This is the first installment based on publicly available materials.- WATCH
May 13, 2022 – TGA Australia: TGA commences evaluation of Moderna COVID 19 vaccine (SPIKEVAX) for children aged 6 months to 5 years old – READ
May 9, 2022 – Case Report Neurology: Acute Transient Encephalopathy after Moderna COVID-19 Vaccine by Rosso et al – READ, PDF, SOURCE
May 4, 2022 – Moderna PRESS RELEASE: Moderna Reports First Quarter 2022 Financial Results and Provides Business Updates – READ
- First quarter 2022 revenues of $6.1 billion
- 2022 signed advance purchase agreements of approximately $21 billion
- Company expects to have four programs in Phase 3 in the second quarter: Omicron-containing bivalent COVID booster, flu, RSV, CMV
May 3, 2022 – PrePrint: Safety and Immunogenicity of a Third Dose of SARS-CoV-2 mRNA Vaccine – An Interim Analysis by Anderson et al – READ. Re Moderna/NIAID Booster arm of Phase 1 trials – HERE & HERE
- Judicial Watch Sues for Records on COVID Vaccine Safety Studies – READ
May 1, 2020 – PRESS RELEASE: Moderna and Lonza Announce Worldwide Strategic Collaboration to Manufacture Moderna’s Vaccine (mRNA-1273) Against Novel Coronavirus – READ, ARCHIVE
- 10-year strategic collaboration agreement to enable larger scale manufacture of Moderna’s mRNA vaccine (mRNA-1273) against the novel coronavirus (SARS-CoV-2) and additional Moderna products in the future.
- The companies plan to establish manufacturing suites at Lonza’s facilities in the United States and Switzerland
April 29, 2022 – PRESS RELEASE: Moderna Finalizes Plan for Long-Term Strategic Partnership with The Government of Canada – today announced its plan to build a state-of-the-art mRNA vaccine manufacturing facility in Quebec that will support a long-term strategic partnership with the Government of Canada to enhance pandemic preparedness – READ
- This milestone follows the signing of a Memorandum of Understanding between Moderna and the Government of Canada in August 2021
- Agreement to produce up to 100 million mRNA respiratory vaccine doses annually [only COVID-19 Wuhan mRNA code went through Phase III clinical trials, and for 3 months only before being unblinded!]
April 19, 2022 – PrePrint: Anti-nucleocapsid antibodies following SARS-CoV-2 infection in the blinded phase of the mRNA-1273 Covid-19 vaccine efficacy clinical trial by Follmann et al – READ, (Sept 2022) Annals of Internal Medicine – HERE, Analysis – READ
- “If the mRNA vaccines decrease the production of anti-nucleocapsid antibodies in a dose dependent fashion, immunity would be short-lived and possibly lessened with additional boosters, the opposite of the desired outcome. This decreased immunity would affect all vaccinated people who had no COVID previous to their vaccination.” – REF
April 8, 2022 – Reuters: Moderna recalls thousands of COVID vaccine doses in Europe – READ
- Moderna recalls 764,900 doses of its COVID-19 vaccine made by its contract manufacturer Rovi after a vial was found contaminated by a foreign body. Lots that were distributed in Norway, Poland, Portugal, Spain and Sweden in January.
April 2022 – TED2022: How mRNA medicine will change the world – Melissa J. Moore, chief scientific officer at Moderna – WATCH, READ, CREDIT, TIMELINE
“It took Moderna’s mRNA design team just one hour to design the mRNA that we immediately put onto our manufacturing equipment.”
Melissa J. Moore PhD, Chief Scientific Officer at Moderna TED2022
“There is a coming Tsunami of mRNA medicine”
Melissa J. Moore
[called vaccines?]
March 29, 2022 – Moderna PRESS RELEASE: Moderna Receives FDA Approval for Emergency Use Authorization of 2nd Booster Dose of Its COVID-19 Vaccine, mRNA-1273 – READ
March 28, 2022 – Moderna has 8 variant vaccines, a COVID+ Flu vaccine and is part of the TeenCOVE and KidCOVE trials for the Wuhan strain in it’s development pipeline – ARCHIVE
March 23, 2022 – Moderna PRESS RELEASE: Moderna Finalizes Strategic Partnership with Australian Government – to establish a state-of-the-art, domestic mRNA vaccine manufacturing facility in Australia, an agreement in principle – READ
March 17, 2022 – The Expose: Exhaustive proof Moderna made Covid-19 [SARS-CoV-2!!] – READ, SGT Report – ARCHIVE, Libety Beacon: Moderna Patented Key Spike Protein Sequence in 2016 – READ
- No virus in nature uses the CGG codon for Arginine in a furin cleavage site.
March 7, 2022 – Moderna’s mRNA Access launched – READ, ARCHIVE
- “At Moderna, we are grounded in the belief that mRNA is transformational. And, for us, our COVID-19 vaccine Spikevax® is just the beginning” – REF

March 7, 2022 – PRESS RELEASE: Moderna Announces Its Global Public Health Strategy – READ, Launching mRNA ACCESS – READ, ARCHIVE
- Commitment to advance vaccines targeting 15 pathogens identified as biggest public health risk by WHO and CEPI into clinical studies by 2025
- Moderna launches mRNA Access, a new collaborative enabling researchers around the world to utilize Moderna’s mRNA technology platform to pursue research in their own labs on emerging and neglected infectious diseases – WEB
- Moderna expands commitment to never enforce COVID-19 patents in the Gavi COVAX AMC for 92 low- and middle-income countries
- Moderna has entered into a Memorandum of Understanding with the Government of the Republic of Kenya to establish its first mRNA manufacturing facility on the continent of Africa
February 4, 2022 – CDC ACIP meeting: Moderna presentation: mRNA 1273 COVID-19 vaccine BLA safety and efficacy data – PDF, SOURCE, ARCHIVE
- “After EUA, subjects were offered unblinding and placebo recipients were offered vaccine”
January 31, 2022 – CNN: Moderna’s Covid-19 vaccine receives full FDA approval – Spikevax for 18 years and over – READ, Associate Press – READ
- “Moderna said the FDA based its decision to give full approval on scientific evidence shared by Moderna in its submission package to the agency, which included six month follow up data from a Phase 3 clinical trial study as well as FDA-required manufacturing and facilities data.”
January 31, 2022 – Moderna PRESS RELEASE: Moderna Receives Full U.S. FDA Approval for COVID-19 Vaccine Spikevax – READ, ARCHIVE, FDA Takes Key Action by Approving Second COVID-19 Vaccine – READ
- “U.S. Food and Drug Administration (FDA) has approved the Biologics License Application (BLA) for SPIKEVAX (COVID-19 Vaccine, mRNA) to prevent COVID-19 in individuals 18 years of age and older.”
- “SPIKEVAX (COVID-19 Vaccine, mRNA) is a vaccine indicated for active immunization to prevent coronavirus disease 2019 (COVID-19) caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in individuals 18 years of age and older.” – note it is not to prevent the virus but the symptoms (disease)!
- “807 million doses of Moderna’s COVID-19 vaccine shipped globally in 2021; approximately 25% of those doses shipped to low- and middle-income countries”
- How could this be following EUA regulatory pathway? – TIMELINE
January 31, 2022 – FDA Press Release: Coronavirus (COVID-19) Update: FDA Takes Key Action by Approving Second COVID-19 Vaccine – ARCHIVE
- Moderna COVID-19 Vaccine; the approved vaccine will be marketed as Spikevax for the prevention of COVID-19 in individuals 18 years of age and older….
- Spikevax has the same formulation as the EUA Moderna COVID-19 Vaccine and is administered as a primary series of two doses, one month apart.
- The Spikevax biologics license application (BLA) builds upon the data and information that supported the EUA, such as preclinical and clinical data, as well as details of the manufacturing process and the sites where the vaccine is made.”
- FDA: SPIKEVAX package Inserts – ARCHIVE
- “Indication: Active immunization against coronavirus disease 2019 (COVID-19) caused by the SARS‑CoV-2 virus in persons 18 years of age and older.”
- [Note how the product immunizes against a disease (i.e. symptoms) and not against the alleged causal agent – the virus called SARS-CoV-2 – words matter!]
- FDA: Spikevax and Moderna COVID-19 Vaccine – Includes press release links as a timeline since 2020 – ARCHIVE
2021
December 17, 2021 – Journal of Investigational Allergology and Clinical Immunology – Letter: Hypersensitivity to the Moderna COVID-19 vaccine caused by tromethamine: PEG is not always the culprit excipient – Rama et al – READ, OTHER
December 13, 2021 – PRESS RELEASE: Moderna and Australia Announce Collaboration to Bring mRNA Manufacturing to Australia – READ,
- Moderna, the Australian Government and the Victorian Government has reached an in-principle agreement to build an mRNA vaccine manufacturing facility in Victoria – READ
November 10, 2021 – Reuters: Germany recommends only Biontech/Pfizer vaccine for under-30s -as it causes fewer heart inflammations in younger people than the Moderna – READ
October 18, 2021 – Fierce Pharma: Pfizer, Moderna will rake in a combined $93 billion next year on COVID-19 vaccine sales: report – READ, CREDIT
December 8, 2021 – ABC News Australia: Moderna vaccine approved by TGA for COVID-19 booster shot in adults – [This is technically misleading as TGA has only Provisionally Approved, not fully approved this booster – the word “registered” and “approved” are used interchangably] – READ
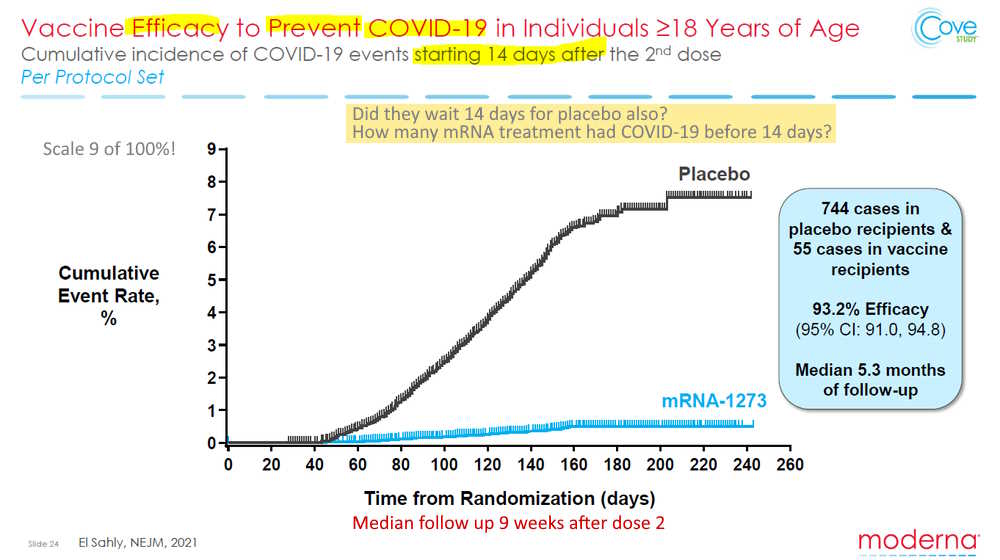
November 4, 2021 – NEJM: Efficacy of the mRNA-1273 SARS-CoV-2 Vaccine at Completion of Blinded Phase by Sahly et al – READ, SUPPLEMENT, An analysis of this paper by Dr Jessica Rose – HERE, Twitter – HERE
“More people died in the drug arm following dose 2 than in the placebo arm”
October 7, 2021 – The Post Millennial: Sweden and Denmark suspend Moderna shots for under 20 year olds after myocarditis study – READ
- The decision to halt the vaccine came after data showed an increase in myocarditis among people under 30, particularly those who received the Moderna shot.
- “The connection is especially clear when it comes to Moderna’s vaccine Spikevax, especially after the second dose,” said the Swedish Ministry of Health in a statement.
October 7, 2021 – Reuters – Sweden, Denmark pause Moderna COVID-19 vaccine for younger age groups – READ
October 6, 2021 – National Pulse: REVEALED: Pfizer Lobbying Hits Decade High as DOZENS of High-Profile Political Appointees Become Big Pharma Reps – [Pfizer & Moderna names] – READ
September 20, 2021 – Judicial Watch, the government watchdog group, announced today that it filed a Freedom of Information Act (FOIA) lawsuit against the Department of Health and Human Services (HHS) for biodistribution studies and related data for the Pfizer, Moderna, and Johnson & Johnson vaccines – READ
- FDA, CDC and NIAID failed to respond to a June 7, 2021, FOIA request for:
- “[A]ccess to biodistribution studies and related data for the Pfizer, Moderna, and Johnson & Johnson vaccines used to treat and/or prevent SARS-CoV-2 and/or COVID-19.”
September 8, 2021 – Business Wire: Resilience to Manufacture mRNA for Moderna’s COVID-19 Vaccine – READ, SOURCE
- National Resilience, Inc. (Resilience), a company seeking to build the world’s most advanced biopharmaceutical manufacturing ecosystem, and Moderna, today announced an agreement to manufacture mRNA for the Moderna COVID-19 vaccine.
August 26, 2021 – Nikkei Asia: 1.6m Moderna doses withdrawn in Japan over contamination – READ, SOURCE
August 12, 2021 – FDA PRESS: Coronavirus (COVID-19) Update: FDA Authorizes Additional Vaccine Dose for Certain Immunocompromised Individuals – Other fully vaccinated individuals do not need an additional vaccine dose right now — Pfizer & Moderna – READ, ARCHIVE
August 10, 2021 – PRESS RELEASE: Moderna and Canada Announce Collaboration to Bring mRNA Manufacturing to Canada – “announced a Memorandum of Understanding (MoU) with the government of Canada to build a state-of-the-art messenger RNA (mRNA) vaccine manufacturing facility in Canada including access to Moderna’s mRNA development engine.” – READ
August 5, 2021 – Gateway Pundit: WOW: Moderna Calls for 3rd Shot of Vaccine to Protect Against New Strains — On Same Day Company Posts $4 Billion 2nd Quarter Profit on Vaccine – READ
- Seattle PI: Moderna COVID-19 vaccine tallies more than $4B in Q2 sales – helping to push the vaccine developer into a profit- ARCHIVE
- “Overall, Moderna earned $2.78 billion in the second quarter, compared to a loss of $117,000 last year, before its vaccine received emergency use authorization in the U.S. and other countries to fight the global pandemic.
- The company brought in $4.35 billion in total revenue, thanks to the vaccine and some grants. Earnings per share totaled $6.46….”
- “The company also said Thursday an analysis showed that its vaccine remains 93% effective as much as six months after the second dose”
July 26, 2021 – ABC News: Australia is racing to make mRNA COVID vaccines here — but can we do it without big pharma? – READ
July 20, 2021 – ICAN: FEDERAL COURT RULES IN ICAN’S FAVOR AND ORDERS COVID-19 SAFETY DATA TO BE DISCLOSED – forces the NIH to unredact and disclose safety-related data they tried to withhold from Moderna’s Phase 1 clinical trial report. – READ, UPDATE, ICAN submitted original FOIA request on May 22, 2020 – HERE
- 322-page Safety Summary Report along with over 700 pages of Appendices to that report, detailing safety data from Moderna’s Phase I clinical trial for its and NIAID’s COVID-19 vaccine. Moderna report – PDF
July 7, 2021 – Letter to the Editor NEJM from Moderna: Durability of Responses after SARS-CoV-2 mRNA-1273 Vaccination – READ, Intrem durability Phase 1 study – submitted Dec. 3, 2020 – READ
June 23, 2021 – ACIP met to discuss myocarditis post mRNA vaccination revealing alarming preliminary statistics in children and young adults – WATCH, TIMELINE, Discussed on The Highwire Ep 221 – EXCERPT
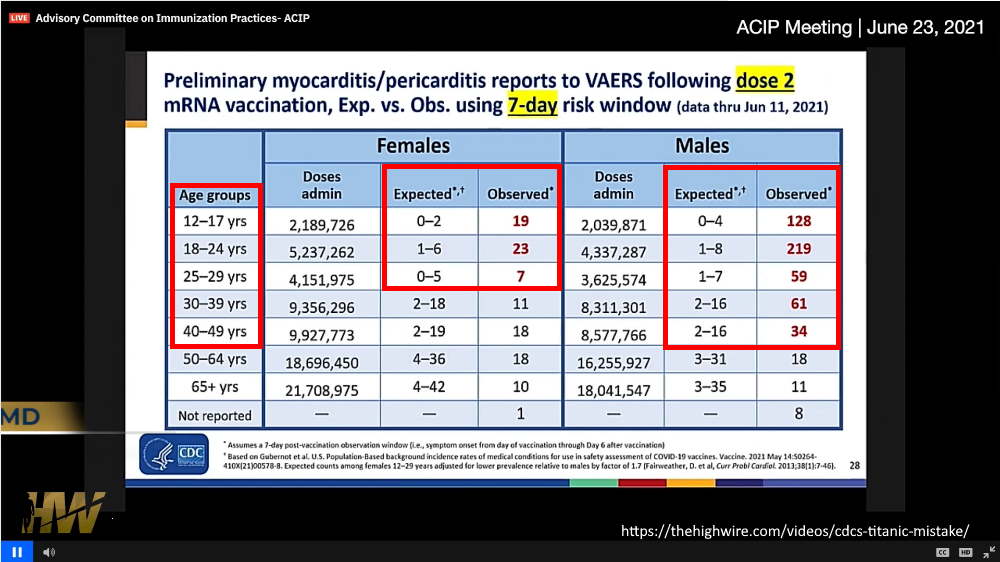
June 22, 2021: Stew Peters Network: BREAKING! Recordings of Moderna Representative Making HORRIFIC Admission About Jab – WATCH @5:30min
June 1, 2021 – NY Post: Moderna applies for full FDA approval of its COVID-19 vaccine – READ
May 13, 2021 – ABC News: Australia signs deal for 25M Moderna doses through next year – READ
April 26, 2021 – Sanofi Press Release: Sanofi to help manufacture Moderna COVID-19 vaccine, supporting global supply demands – Australia, Queensland – READ, CREDIT
April 7, 2021 – Gardian: Welsh patients to be first in UK to receive Moderna Covid vaccine – READ
February 9, 2021 – PRESS RELEASE: Moderna Announces COVID-19 Vaccine Supply Agreements with the Government of Taiwan for 5 Million Doses and the Government of Colombia for 10 Million Doses – deliveries would begin in mid-2021 pending regulatory approval – READ
February 4, 2021 – NEJM: Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine – Moderna (submitted Dec. 31, 2020) – READ
- Phase 3 randomized, observer-blinded, placebo-controlled trial was conducted at 99 centers across the United States.
- The primary end point was prevention of Covid-19 illness with onset at least 14 days after the second injection in participants who had not previously been infected with SARS-CoV-2.
- “The COVE trial provides evidence of short-term efficacy of the mRNA-1273 vaccine in preventing symptomatic SARS-CoV-2 infection in a diverse adult trial population.”
- Conclusion: “The mRNA-1273 vaccine showed 94.1% efficacy at preventing Covid-19 illness, including severe disease.”
February 3, 2021 – PRESS RELEASE: Singapore Health Sciences Authority (HSA) Approves Interim Authorization of COVID-19 Vaccine Moderna For Use – Singapore Health Sciences Authority (HSA) has approved the interim authorization under its Pandemic Special Access Route (PSAR). Govt supply agreement announced on December 14, 2020 – READ, ARCHIVE
January 29, 2021 – CDC | MMWR 70(4): Allergic Reactions Including Anaphylaxis After Receipt of the First Dose of Moderna COVID-19 Vaccine — United States, December 21, 2020–January 10, 2021 – READ
January 27, 2021 – PRESS RELEASE: Moderna Confirms Discussions with U.S. Government for Additional 100 Million Doses of the Moderna COVID-19 Vaccine – bringing their confirmed commitmment to 300 million doses – READ, READ
January 25, 2021 – PRESS RELEASE: Moderna COVID-19 Vaccine Retains Neutralizing Activity Against Emerging Variants First Identified in the U.K. and the Republic of South Africa – READ, ARCHIVE, BOOSTER trials to start looking at both current version and variant candidates as “waning immunity” signals have appeared with Beta variant. – TIMELINE
- The in vitro study assessed the ability of mRNA-1273 to elicit potently neutralizing antibodies against new SARS-CoV-2 variants, using sera from eight Phase 1 clinical trial participants (aged 18-55 years) who received two 100 µg doses of mRNA-1273, and separately using sera from non-human primates (NHPs) immunized with two doses of 30 µg or 100 µg of mRNA-1273
- Emerging variants tested, including B.1.1.7 [September 2020, UK – Alpha, with 17 total mutations, 8 located in the spike protein] and B.1.351 [Republic of South Africa – Beta] – WHO variants – HERE
- The NIAID led study showed no significant impact on neutralizing titers against the B.1.1.7 variant relative to prior variants but a 6-fold reduction in neutralizing titers observed with the B.1.351 variant relative to prior variants. “These lower titers may suggest a potential risk of earlier waning of immunity to the new B.1.351 strains.
- The 2 x 100 µg dose is “expected to be protective against emerging strains detected to date”, but start on trialing booster candidates “out of an abundance of caution”
“…we believe it is imperative to be proactive as the virus evolves… Out of an abundance of caution and leveraging the flexibility of our [never used before 2020 human trials!] mRNA platform, we are advancing an emerging variant booster candidate against the variant first identified in the Republic of South Africa into the clinic to determine if it will be more effective to boost titers against this and potentially future variants.”
Stéphane Bancel, Moderna CEO
Moderna announced they will:
- First, “test an additional booster dose “of its current COVID-19 Vaccine (mRNA-1273) to study the ability to further increase neutralizing titers against emerging strains beyond the existing primary vaccination series.
- Second, they would begin “an emerging variant booster candidate (mRNA-1273.351) against the B.1.351 variant…into preclinical studies and a Phase 1 study in the U.S. to evaluate the immunological benefit of boosting with strain-specific spike proteins.
- Moderna expects either one will “further boost neutralizing titers in combination with all of the leading vaccine candidates.”
January 21, 2021 – PRESS RELEASE: Moderna Announces First Participant Dosed in Phase 1/2 Study of Moderna COVID-19 Vaccine in Japan Led by [business partner] Takeda Pharmaceutical – – READ, ClinicalTrials.gov identifier is NCT04677660 – READ, ARCHIVE
- This is the first clinical trial of a Moderna product in Japan, using placebo or a 100 μg dose, 28 days apart with 200 participants, 20yrs+
- “Participants will be followed through 12 months after the second vaccination”. Japan has commited to 50 million doses
January 18, 2021 – McKesson (Distributor) Vaccine Distribution Updates: Moderna COVID-19 Vaccine Distribution Delay – ARCHIVE
- “certain deliveries of Moderna vaccines shipped on Sunday, Jan. 17, arrived at the sites of administration colder than the low end of the manufacturer’s stated temperature range. McKesson is replacing these vaccines.”
- “McKesson is honored to play a pivotal role in this initiative of unprecedented scale. In this complex distribution program,…”
January 14, 2021 – Best News: The Moderna CEO Just Made This Scary Prediction About COVID – Moderna CEO Stephane Bancel believes COVID will be with us “forever,” becoming an endemic disease. – READ, Yet that was already predicted in July 2020 – REF
January 12, 2021 – PRESS RELEASE: Swissmedic Authorizes COVID-19 Vaccine Moderna for Use in Switzerland – READ– Swismedic, the Swiss Agency for Therapeutic Products – The authorization is given according to the ordinary approvals procedure and is based on a rolling submission of data. The govt. has secured 7.5 million doses. [This is increased to 13.5 million by Feb. 3, 202, as talk of boosters escalates – READ]
“Switzerland has played a critical role in Moderna’s history since our early days …”
Stéphane Bancel
January 11, 2021 – PRESS RELEASE: Moderna Provides Business Update and Announces Three New Development Programs in Infectious Disease Vaccines – READ, ARCHIVE
“The uniquely challenging year of 2020 for all of society proved to be an extraordinary proof-of-concept period for Moderna…
Moderna “today announced that it is expanding its pipeline of innovative vaccines with three new development programs based on the clinical success of its infectious disease vaccine portfolio to date [They’ve only had COVID-19 emergency use authroised!] This announcement reflects the Company’s commitment to accelerating its infectious disease portfolio based on Moderna’s experience with its COVID-19 vaccine. The development programs announced today are mRNA vaccine candidates against seasonal flu, HIV and the Nipah virus” and ” respiratory syncytial virus (RSV) vaccine program into older adults”.
Stéphane Bancel, Moderna CEO
January 8, 2021 – PRESS RELEASE: United Kingdom Medicines and Healthcare products Regulatory Agency Authorizes Use of COVID-19 Vaccine Moderna – UK’s Medicines and Healthcare products Regulatory Agency (MHRA) has approved Moderna’s vaccine for use under Regulation 174. The temporary authorization permits the supply of COVID-19 Vaccine Moderna in Great Britain and is based upon the advice of the UK Commission on Human Medicines. – READ, ARCHIVE
January 6, 2021 – PRESS RELEASE: European Commission Authorizes COVID-19 Vaccine Moderna in Europe – READ, Conditional Marketing Authorisation (CMA) based on recommendation from European Medicines Agency (EMA) “for use of the COVID-19 Vaccine Moderna for active immunization to prevent COVID-19 caused by SARS-CoV-2 virus in individuals 18 years of age and older” – READ, ARCHIVE
January 2021 – The European Medicines Agency (EMA) | Conditional marketing authorisation: Moderna Annex 1: SUMMARY OF PRODUCT CHARACTERISTICS – ARCHIVE, Black Triangle – SOURCE
January 4, 2021 – PRESS RELEASE: Israel’s Ministry of Health (MOH) has given authorization to import the COVID-19 Vaccine Moderna in Israel – READ, ARCHIVE
- This is the third regulatory authorization for the COVID-19 Vaccine Moderna, the first in United States on December 18, 2020 and Canada on December 23, 2020 stated CEO – Isreael secured 6 million doses
- Additional authorizations are currently under review in the European Union, Singapore, Switzerland and the United Kingdom.
- “The authorization is given according to Regulation 29 (A)(9): Medical product designated for pharmaceutical treatment of local citizens in case of epidemic or contagious disease …of the Pharmacists’ Regulations (Medical preparations) – 1986.”
- “The decision from the MOH is based on a rolling submission of data and is based on the totality of scientific evidence shared by the Company, including a data analysis from the pivotal Phase 3 clinical study announced on November 30, [2020]”
January 4, 2021 – PRESS RELEASE: Moderna Provides COVID-19 Vaccine Supply Update – Moderna said it is continuing to invest and add staff to build up to potentially 1 billion doses for 2021 – READ
2020
December 31, 20202 – PRESS RELEASE: Moderna Announces Publication of Results from the Pivotal Phase 3 Trial of the Moderna COVID-19 Vaccine in The New England Journal of Medicine – READ, ARCHIVE – NEJM published Feb 4, 2021 – PAPER
- The “interim safety and primary efficacy results from the Phase 3 trial of the Moderna COVID-19 Vaccine (mRNA-1273) were published in the New England Journal of Medicine.
- The 100 μg two-dose regime of the Moderna COVID-19 Vaccine given 28 days apart was well-tolerated and demonstrated vaccine efficacy of 94.1% against COVID-19″ [the symptoms]
- The Phase 3 study, known as the COVE study, enrolled more than 30,000 participants in the U.S. and is conducted in collaboration NIAID and BARDA
December 31, 2020 – PRESS RELEASE: Moderna Confirms 40 Million COVID-19 Vaccine Dose Supply Agreement with the Government of the Republic of Korea – not yet approved – READ, READ2
- Moderna’s 2020 vaccine development timline – ARCHIVE

December 25, 2020 – NY Post: Doctor reportedly has severe allergic reaction to Moderna COVID vaccine – Dr. Hossein Sadrzadeh, a geriatric oncologist at Boston Medical Center, suffered a serious allergic reaction to Moderna’s coronavirus vaccine, the first of its kind documented. He became dizzy and felt his heart racing minutes after receiving the vaccine – READ, New York Times – READ
December 23, 2020 – PRESS RELEASE: Health Canada Authorizes Moderna COVID-19 Vaccine in Canada – under an Interim Order – READ Based on a rolling review of data of Phase 3 COVE stody that was announced on October 12, 2020. Canada has confirmed order commitment to 40 million doses.
December 19, 2020 – PRESS RELEASE: U.S. CDC Advisory Committee on Immunization Practices [ACIP] Recommends Vaccination with Moderna’s COVID-19 Vaccine for Persons 18 Years and Older – READ, ARCHIVE
December 18, 2020 – PRESS RELEASE: Moderna Announces FDA Authorization of Moderna COVID-19 Vaccine in U.S. – under an Emergency Use Authorization (EUA) – READ, ARCHIVE
- Moderna will continue to gather additional data and plans to file a Biologics License Application (BLA) with the FDA requesting full licensure in 2021
December 18, 2020 -Media Blackout: Moderna’s FDA Report Lists 13 Total Deaths: 6 In The Vaccine Group 7 In The Placebo – WATCH
- “In this report, we examine discrepancies in the FDA Moderna report that was voted on by an advisory panel. The panel voted 20-0 recommending EUA.
- Some of the discrepancies include cherry picked trial participants to achieve the desired results to gain EUA. As well as 13 total deaths in the trials, 6 in the vaccinated group and 7 in the placebo. Something the media refuses to address.”
December 18, 2020 – PRESS RELEASE: European Commission Exercises Option for Additional 80 Million Doses of Moderna’s COVID-19 Vaccine Candidate – bringing total to 160 million doses to date – READ
December 17, 2020 – CNBC: You can’t sue Pfizer or Moderna if you have severe Covid vaccine side effects. The government likely won’t compensate you for damages either – under the PREP Act companies have total immunity from liability – READ
December 17, 2020 – NEJM: Safety and Immunogenicity of SARS-CoV-2 mRNA-1273 Vaccine in Older Adults – Anderson et al Moderna – READ, Sept 2020 – PRESS
December 17, 2020 – PRESS RELEASE: Moderna Receives FDA Advisory Committee [VRBPAC] Vote Supporting Emergency Use for Moderna’s Vaccine Against COVID-19 in the United States – READ, ARCHIVE
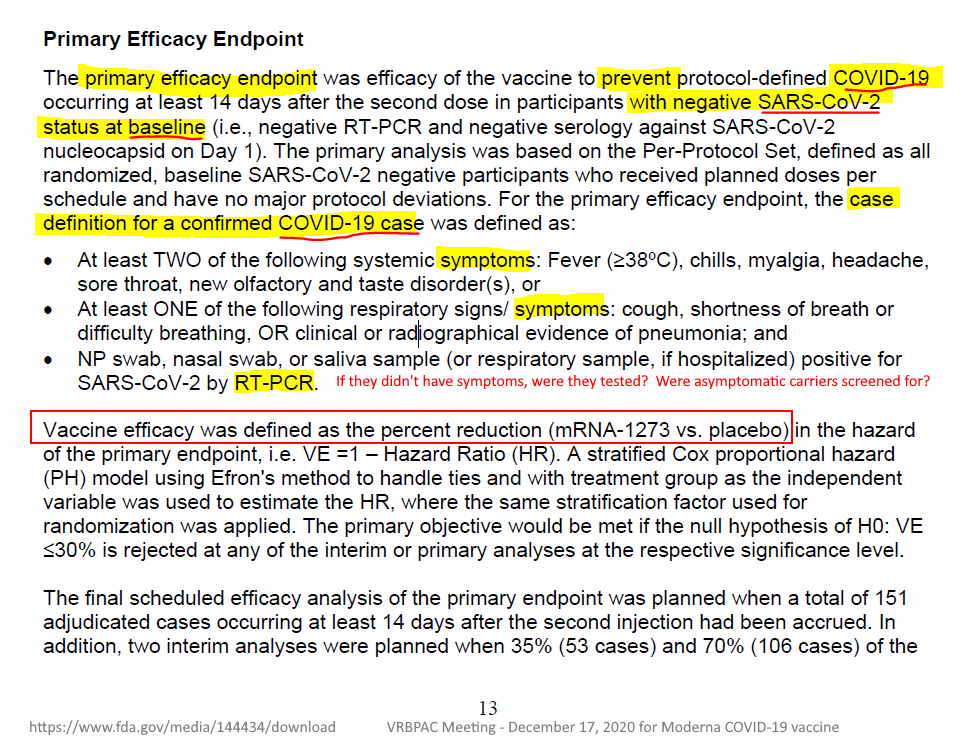
December 17, 2020 – FDA Briefing Document for Moderna COVID-19 Vaccine, Sponsor: ModernaTX, Inc.: Vaccines and Related Biological Products Advisory Committee (VRBPAC) Meeting – READ, ARCHIVE
December 16, 2020 – The Post Millennial – Moderna vaccine slated for FDA approval after being found 94 percent effective – The findings bring the two-dose vaccine closer to final approval for the general population, which is expected to happen when an independent advisory board to the FDA takes up the review on Thursday. – READ
- “The FDA confirmed on Tuesday that the Moderna vaccine is safe and effective for use in adults, clearing the way for FDA approval for emergency use.”
December 15, 2020 – Politico: FDA clears path for second coronavirus vaccine, from Moderna – READ
“Both shots use mRNA technlogy, an approach that sends instructions to cells to produce the antibodies to fight Covid-19 infection. Moderna’s is 94 percent effective after the second dose and especially effective against severe illness, according to the data, which indicate that all 30 cases of severe coronavirus occured in the placebo arm of the trial and none in the vaccine arm.”
[MISINFORMATION by Politico: the mRNA codes for a spike protein antigen, not an instruction “to produce the antibodies”]
December 14, 2020 – PRESS RELEASE: Moderna Confirms Supply Agreement with the Ministry of Health to Supply Singapore with mRNA Vaccine Against COVID-19 (mRNA-1273) – READ – [doses not mentioned]
- “Moderna is scaling up global manufacturing to be able to deliver approximately 500 million doses per year and possibly up to 1 billion doses per year, beginning in 2021″
- On the back of recent announcement that the vaccine “remains stable at 2° to 8°C (36° to 46°F), the temperature of a standard home or medical refrigerator, for 30 days.”
December 11, 2020 – PRESS RELEASE: Moderna Announces First Adolescent Dosed in Phase 2/3 Study of COVID-19 Vaccine Candidate [mRNA-1273] in Adolescents – study expected to enroll 3,000 healthy adolescents ages 12 to less than 18 to receive two doses of either placebo or a 100 μg vaccine. 12 month follow through – READ, ARCHIVE, The ClinicalTrials.gov identifier is NCT04649151 – collaboration with BARDA – READ, ARCHIVE
December 8, 2020 – PRESS RELEASE: Switzerland Exercises Increased Option for 7.5 Million Doses of mRNA Vaccine Against COVID-19 (mRNA-1273) – READ
December 7, 2020 – PRESS RELEASE: Canada Exercises Increased Option for Total of 40 Million Doses of mRNA Vaccine Candidate Against COVID-19 (mRNA-1273) – READ, ARCHIVE
- “The Canadian vaccine supply will be sourced from Moderna’s European production capacity with its strategic manufacturing partner Lonza in Switzerland, and ROVI in Spain for fill-finish services.”
December 4, 2020 – PRESS RELEASE: Moderna Announces Amendment to Supply Agreement with the Ministry of Health of Israel to Supply Additional Doses of mRNA Vaccine Against COVID-19 (mRNA-1273) – READ, ARCHIVE
December 3, 2020 – PRESS RELEASE: Moderna Provides Updates on the Clinical Development and Production of Its COVID-19 Vaccine Candidate – [Data by press release] – READ, ARCHIVE
- “Interim durability data from NIH-led Phase 1 study of mRNA-1273 published as letter to the editor in NEJM; at day 119, 3 months post-second 100 μg dose, binding and neutralizing antibody titers remain high in all participants; results consistent across all age groups (18-55, 56-70 and 71+)” – “science” by press release – NEJM – CORRESPONDENCE
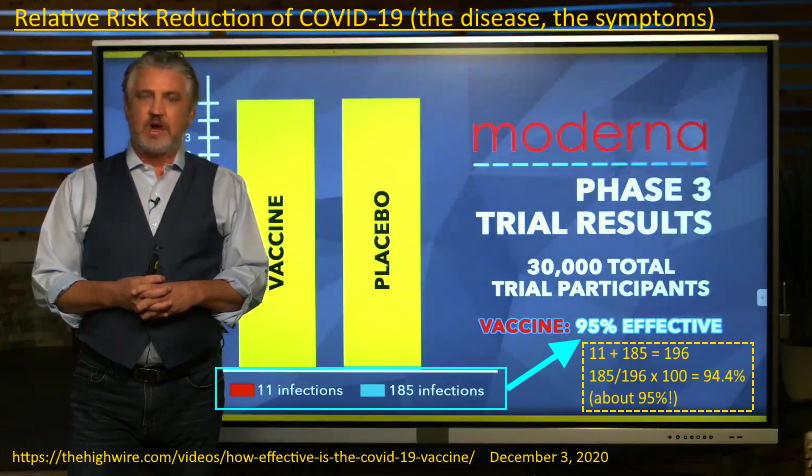
December 3, 2020 – The Highwire: HOW EFFECTIVE IS THE COVID-19 VACCINE? – WATCH
- 95% effective at preventing your symptoms, the disease called COVID-19, not infection of the virus called SARS-CoV-2. The clinical trails were not designed to test for preventing infection or looking at whether the vaccine prevented transmission.
November 30, 2020 – PRESS RELEASE: Moderna Announces Primary Efficacy Analysis in Phase 3 COVE Study for Its COVID-19 Vaccine Candidate and Filing Today with U.S. FDA for Emergency Use Authorization – “Vaccine efficacy against COVID-19 was 94.1%; vaccine efficacy against severe COVID-19 was 100%” – ARCHIVE, Science News – ARTICLE
- This announcement spurs on additional vaccine orders by many countries in the weeks ahead
November 29, 2020 – PRESS RELEASE: Moderna Announces Amendment to Current Supply Agreement with United Kingdom Government for an Additional 2 Million Doses of mRNA Vaccine Against COVID-19 (mRNA-1273) – READ, ARCHIVE
- “On November 16, Moderna announced that the independent, NIH-appointed Data Safety Monitoring Board (DSMB) for the Phase 3 study of mRNA-1273, its vaccine candidate against COVID-19, has informed Moderna that the trial has met the statistical criteria pre-specified in the study protocol for efficacy, with a vaccine efficacy of 94.5%.”
November 25, 2020 – PRESS RELEASE: Moderna Announces the European Commission’s Approval of Advance Purchase Agreement for Initial 80 Million Doses of mRNA Vaccine Against COVID-19 (mRNA-1273) – Agreement reflects Moderna’s commitment to make its vaccine available in multiple countries – READ, ARCHIVE
November 24, 2020 – Independent UK: Vaccines may not prevent the transmission of COVID-19 from vaccinated to unvaccinated, Moderna chief doctor warns – READ, Axios – READ
- Moderna vaccine showed “94.5% effective at protecting people from COVID-19 (the symptoms) this news doesn’t necessarily mean those who are vaccinated will be unable to transmit the virus (SARS-CoV-2) to the unvaccinated.
- [The products need to be trialled in the population to see if they work as a “vaccine” is believed to work, that is at stoping the spread of the virus! In the mean time we’ll use it to prevent sickness!]
I think we need to be careful, as we get vaccinated, not to over-interpret the results. Our results show that this vaccine can prevent you from being sick, prevent you from being severely sick. They do not show that they prevent you from potentially carrying this virus transiently and infecting others.
When we start the deployment of this vaccine we will not have sufficient, concrete data to prove that this vaccine reduces transmission.
Do I believe it reduces transmission, absolutely yes, I say this because of The Science. But absent proof, I think it’s important that we don’t change behaviours solely on the basis of vaccination.
Dr Tal Zaks, Moderna
November 23, 2020 – Axios: Moderna exec says children could be vaccinated by mid-2021 – READ
November 18, 2020 – The Guardian: Rishi Sunak refuses to say if he will profit from Moderna Covid vaccine – Chancellor Sunak’s former hedge fund ( Theleme fund, registered in the Cayman Islands of which Sunak was a founding partner) invested heavily in Moderna – READ
November 17, 2020 – PRESS RELEASE: Moderna Announces Supply Agreement with United Kingdom Government to Supply mRNA Vaccine Against COVID-19 (mRNA-1273) if Approved for Use – READ, ARCHIVE
November 17, 2020 – PRESS RELEASE: European Medicines Agency Begins Rolling Review of Moderna’s mRNA Vaccine Candidate Against COVID-19 (mRNA-1273) – READ
November 16, 2020 – Crunchbase: With Flagship Behind It, Moderna Quickly Scaled From Startup To World-Changing Biotech – READ
- “Moderna shook the world this morning with the announcement that its COVID-19 vaccine was 94.5 percent effective in Phase 3 trials….Moderna shares jumped 10 percent, landing the company a market capitalization around $39 billion.”
- Dr. Anthony Fauci…declared the Moderna trial outcomes “as good as it gets.”
- “The company that accomplished this [Moderna] is itself little more than a startup.”!!
November 16, 2020 – The Week: Fauci: Moderna’s ‘outstanding’ vaccine results are ‘as good as it gets‘ – READ
- These are obviously very exciting results,” Fauci told CNN on Monday. “It’s just as good as it gets — 94.5 percent is truly outstanding.” – TWEET
November 16, 2020 – PRESS RELEASE: Moderna’s COVID-19 Vaccine Candidate Meets its Primary Efficacy Endpoint in the First Interim Analysis of the Phase 3 COVE Study – READ, PDF, ARCHIVE, CLINICAL STUDY PROTOCOL 135 pages – PDF
- First Phase III interim analysis included 95 participants with confirmed cases of COVID-19 met statistical criteria with a vaccine efficacy of 94.5% (p <0.0001)
- Moderna intends to submit for an Emergency Use Authorization (EUA) with U.S. FDA in the coming weeks and expects the EUA to be based on the final analysis of 151 cases and a median follow-up of more than 2 months
- MODERNA CLINICAL STUDY PROTOCOL – A Phase 3, Randomized, Stratified, Observer-Blind, Placebo-Controlled Study to Evaluate the Efficacy, Safety, and Immunogenicity of mRNA-1273 SARS-CoV-2 Vaccine in Adults Aged 18 Years and Older (mRNA-1273-P301) – 135 pages – (Aug 20, 2020 version) – READ, ARCHIVES
November 16, 2020 – PRESS RELEASE: Moderna Announces Longer Shelf Life for its COVID-19 Vaccine Candidate at Refrigerated Temperatures – “Vaccine candidate now expected to remain stable at standard refrigerator temperatures of 2° to 8°C (36° to 46°F) for 30 days, up from previous estimate of 7 days” – ARCHIVE
November 16, 2020 – Science News: Moderna says its COVID-19 vaccine is nearly 95 percent effective – Two leading candidates have now reported preliminary data showing success in preventing illness – READ
November 16, 2020 – PRESS RELEASE: Moderna’s COVID-19 Vaccine Candidate Meets its Primary Efficacy Endpoint in the First Interim Analysis of the Phase 3 COVE Study – ARCHIVE
- First interim analysis included 95 participants with confirmed cases of COVID-19
- Phase 3 study met statistical criteria with a vaccine efficacy of 94.5% (p <0.0001)
November 13, 2020 – PRESS RELEASE: Swissmedic Begins Rolling Review of Moderna’s mRNA Vaccine Against COVID-19 (mRNA-1273) – READ
November 12, 2020 – NEJM: An mRNA Vaccine against SARS-CoV-2 — Preliminary Report – Jackson et al – Moderna study group Phase 1 clinical trial report – READ Clinical trail NCT04283461 – HERE, Protocol – PFD, Supplementary Appendix – PDF
(announced Press Release July 14, 2020 – HERE) The Highwire – WATCH (this is the correct paper not the title they show)
- Phase 1, dose-escalation, open-label trial including 45 healthy adults 18 to 55 years of age, who received two vaccinations, 28 days apart, with mRNA-1273 in a dose of 25 μg, 100 μg, or 250 μg. There were 15 participants in each dose group.
- “Solicited adverse events that occurred in more than half the participants included fatigue, chills, headache, myalgia, and pain at the injection site. Systemic adverse events were more common after the second vaccination, particularly with the highest dose”
- “Seroconversion was rapid for binding antibodies occurring within 2 weeks after the first vaccination, but pseudovirus neutralizing activity was low before the second vaccination, which supports the need for a 2-dose vaccination schedule.”
- First shot creates binding antibodies – which could lead to immune enhancement problem
- Second shot was “needed” to get the disired neutralising antibodies
- “Though correlates of protection from SARS-CoV-2 infection have not yet been determined, measurement of serum neutralizing activity has been shown to be a mechanistic correlate of protection for other respiratory viruses, such as influenza” [The vaccine is rolled out in Dec 2020! and they don’t know!)
October 29, 2020 – PRESS RELEASE: Moderna Partners with Takeda and the Government of Japan to Supply 50 Million Doses of mRNA Vaccine Against COVID-19 (mRNA-1273) to Japan – READ
October 27, 2020 – PRESS RELEASE: UK Medicines and Healthcare products Regulatory Agency Begins Rolling Review of Moderna’s mRNA Vaccine Against COVID-19 (mRNA-1273) – as phase III clinical trial enrolment has completed – READ
October 26, 2020 – PRESS RELEASE: Moderna Announces Supply Agreement with the Ministry of Public Health to Supply Qatar with mRNA Vaccine Against COVID-19 (mRNA-1273) – READ, ARCHIVE
October 22, 2020 – PRESS RELEASE: Moderna Completes Enrollment of Phase 3 COVE Study of mRNA Vaccine Against COVID-19 (mRNA-1273) – READ, ARCHIVE
- Moderna separately shared its statement at today’s U.S. Food and Drug Administration (FDA) Vaccines and Related Biological Products Advisory Committee (VRBPAC) meeting. – HERE, WATCH
October 15, 2020 – NEJM: Evaluation of the mRNA-1273 Vaccine against SARS-CoV-2 in Nonhuman Primates – Corbett et al (Moderna) – READ (submitted July 28, 2020 – PRESS, ARCHIVE)
- “Postchallenge humoral S- and N-specific IgG increased in control animals within 2 weeks after challenge, whereas antibody levels in mRNA-1273–vaccinated animals remained stable; thus, no anamnestic response was found after challenge”
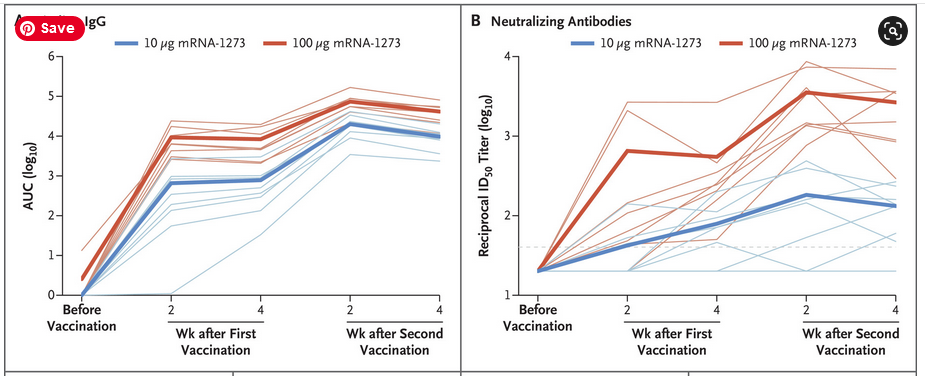
- High production of binding antibodies is not good, they bind to the virus, but are not cleared from the body. They could make the recipient prone to antibody dependent enhancement.
October 14, 2020 – PRESS RELEASE: Moderna Receives Confirmation of Eligibility for Submission of Marketing Authorization Application to the European Medicines Agency for mRNA Vaccine Against COVID-19 (mRNA-1273) – READ
October 13, 2020 – PRESS RELEASE: Moderna Announces Initiation of Rolling Submission to Health Canada for mRNA Vaccine Against COVID-19 (mRNA-1273) – READ, ARCHIVE
October 8, 2020 – PRESS RELEASE: DARPA Awards Moderna up to $56 Million to Enable Small-Scale, Rapid Mobile Manufacturing of Nucleic Acid Vaccines [mRNA] and Therapeutics – READ
September 29, 2020 – PRESS RELEASE: Moderna Announces Publication in The New England Journal of Medicine of Interim Results From Older Adult Age Cohorts in Phase 1 Study of its mRNA Vaccine Against COVID-19 (mRNA-1273) – (submitted today) READ, PDF, ARCHIVE, NEJM (pub Dec. 17, 2020) – PAPER
- mRNA-1273 induced consistently high levels of pseudovirus neutralization antibody titers in all participants in the 56-70 (n=10) and 71+ (n=10) age cohorts etc
- At the 25 µg and 100 µg dose levels, mRNA-1273 was generally well-tolerated in all age cohorts
September 22, 2020 – PRESS RELEASE: Canada Exercises Increased Option for 20 Million Doses of mRNA Vaccine Against COVID-19 (mRNA-1273) – READ
Septebmer 16, 2020 – PRESS RELEASE: Moderna Announces First Commercial Organization Outside North America in Switzerland – READ
- Switzerland, headed by Dan Staner as Vice President and General Manager, is the first country outside of North America to host a Moderna regional hub and commercial organization
Septebmer 16, 2020 – PRESS RELEASE: Moderna Names Michael Mullette as Managing Director of New Canadian Subsidiary – permanent expansion to Moderna’s North America footprint at a time when the company is scaling up operations for late-stage development and large scale manufacturing of mRNA-1273 – READ
September 8, 2020 – PRESS RELEASE: Biopharma Leaders Unite to Stand with Science – READ, Redirection READ, ARCHIVE, TIMELINE
- September 8, 2020 – CNN: 9 vaccine makers sign safety pledge in race for COVID-19 vaccine – READ, NPR – READ, BBC- READ
August 28, 2020 – PRESS RELEASE: Moderna Confirms Discussions with the Ministry of Health, Labour and Welfare to Supply Japan with 40 Million Doses of mRNA Vaccine Against COVID-19 (mRNA-1273) – READ
August 26, 2020 – PRESS RELEASE: Moderna to Present New Interim Clinical Data About mRNA Vaccine Against COVID-19 (mRNA-1273) at Advisory Committee on Immunization Practices (ACIP) Meeting – READ
August 24, 2020 – PRESS RELEASE: Moderna Confirms Advanced Discussions with European Commission to Supply Europe with 80 Million Doses of mRNA Vaccine Against COVID-19 (mRNA-1273) – READ
August 11, 2020 – PRESS RELEASE: Moderna Announces Supply Agreement with U.S. Government for Initial 100 Million Doses of mRNA Vaccine Against COVID-19 (mRNA-1273) – READ
August 10, 2020 – PRESS RELEASE: John Lepore [ex GSK] Joins Moderna as Senior Vice President, Government Engagement – READ
August 6, 2020 – The Highwire Ep 175: CORONAVIRUS: THE FRONTLINE PUSHBACK -The Jaxen Reeport: Moderna executives cash in shares and make millions! – [@32:23] WATCH, They used 10b5-1 insider trading form – for insiders who poses material non-public information – REF
- Moderna is America’s #1 choice for COVID-19 vaccine
- Stephane Bancel became CEO of Moderna in 2011 and owns a roughly 8% stake in the publicly traded company – READ, he became a billionaire for the first time April 2020 – READ
- Dr Elizabeth Nabel – resignes over trial Conflict of Interest – READ
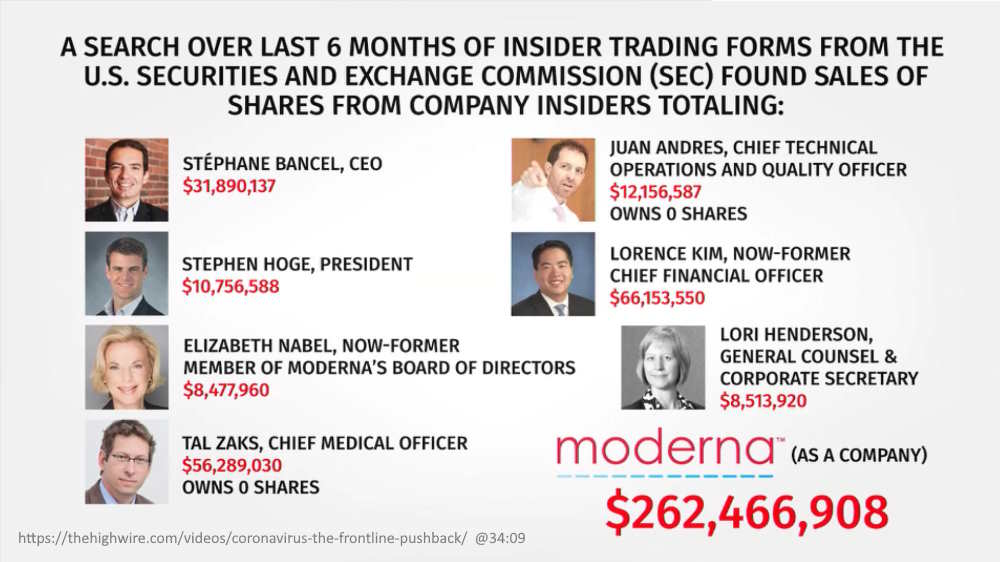
August 5, 2020 – PRESS RELEASE: Moderna Reports Second Quarter 2020 Financial Results and Provides Business Updates – READ
July 30, 2020 – Boston Globe: Brigham president resigns from Moderna board after conflict of interest questions raised – Dr. Elizabeth Nabel’s hospital is participating in the Cambridge biotech’s trial of a COVID-19 vaccine. She recently sold stock in it worth nearly $6.5m – READ, Stat News – READ, CREDIT
- “Dr. Elizabeth Nabel, president of Brigham and Women’s Hospital, said Thursday she was resigning from the Moderna board of directors after the Globe inquired about whether her position at [Moderna] conflicted with her hospital’s leadership role in a large study of Moderna’s experimental COVID-19 vaccine.”
- Elisabeth “Betsy” “Nabel ― who is also a cardiologist and professor at Harvard Medical School ― last year received $425,000 in stock option awards from Moderna, as well as $62,500 in payment, according to a filing with the Securities and Exchange Commission. The 10-year-old biotech’s share price took off after the company announced in February it had made its first batch of the vaccine candidate.”
July 28, 2020 – PRESS RELEASE: Moderna Announces Publication in The New England Journal of Medicine of Non-Human Primate Preclinical Viral Challenge Study of its mRNA Vaccine Against COVID-19 (mRNA-1273) – READ, ARCHIVE, NEJM – STUDY, Moderna’s timeline – READ
- mRNA-1273 led to protection against SARS-CoV-2 infection in the lungs and nose of non-human primates
- No evidence of vaccine-associated enhanced disease (VAERD) observed [Pathogenic Priming!]
- Moderna currently has nine development candidates in its prophylactic vaccines modality….including COVID-19 – READ
- BARDA federal funding under Contract no. 75A50120C00034 – LINK
July 28, 2020: NEJM: Evaluation of the mRNA-1273 Vaccine against SARS-CoV-2 in Nonhuman Primates -Corbett et al Moderna – ARCHIVE, peer reviewd Oct 15, 2020 – READ
July 27, 2020 – EurekAlert: Brigham takes on leadership role in COVID-19 vaccine trials – Brigham and Women’s Hospital will be the clinical trial site in New England for the COVE Study, with Dr. Lindsey Baden serving as co-principal investigator – READ, Brigham and Women’s Hospital presser – READ, CREDIT
- The president of Brigham and Women’s Hospitals, Dr Elizabeth “Betsy” Nabel is on the Moderna board of directors!
- She resigned from Moderna board ONLY AFTER challenged by The Boston Globe…ethically this should have been before! Given that she has cashed in on Moderna stocks (~$8.5 million) she should have resigned from the hospital where the trial is being run.
- The hospital froze Executive wages, which in light of Betsy’s payout she could “afford” to stay! – ~@40min CREDIT
- “The COVE study is part of Operation Warp Speed (OWS), which aims to deliver 300 million doses of a safe, effective vaccine for COVID-19 by early 2021″
- “A preliminary report on data from the phase 1 trial of mRNA-1273 was recently published [see July 14, 2020] in the New England Journal of Medicine, which found evidence that the vaccine induced an anti-SARS-CoV-2 immune response in all 45 participants.”
July 27, 2020 – The Hill: First phase 3 test of coronavirus vaccine candidate begins in US for Moderna – 30,000 participants expected – READ
- BARDA has provided total of $955 million through operation warp speed – HHS – READ, ARCHIVE
- Working with Coronavirus Prevention Network – ARCHIVE, Initial page – HERE, Clinical Trial Phases explained – HERE
July 27, 2020 – PRESS RELEASE: Moderna Announces Phase 3 COVE Study of mRNA Vaccine Against COVID-19 (mRNA-1273) Begins in US with 30,000 expected participants in collaboration with NIH & BARDA – ARCHIVE
July 27, 2020 – NIH New Release: Phase 3 clinical trial of investigational vaccine for COVID-19 begins – Multi-site trial to test candidate developed by Moderna and NIH. – READ, ARCHIVE, CREDIT
- ClinicalTrials.gov and search identifier NCT04470427, launched July 14, 2020
- The COVID-19 Prevention Network (CoVPN) was formed by the National Institute of Allergy and Infectious Diseases (NIAID) at the US National Institutes of Health (NIH) to respond to the global pandemic.- They will be conducting the Moderna clinical trials – seeking volunteers – ARCHIVE, TIMELINE
- The Moderna “trial is designed to evaluate the safety of mRNA-1273 and to determine if the vaccine can prevent symptomatic COVID-19 after two doses. As secondary goals, the trial also aims to study whether the vaccine can prevent severe COVID-19 or laboratory-confirmed SARS-CoV-2 infection with or without disease symptoms. The trial also seeks to answer if the vaccine can prevent death caused by COVID-19 and whether just one dose can prevent symptomatic COVID-19″ [note the disease “or” infection, not both!]
July 26, 2020 – Aljazeera: Moderna gets another $472m in US gov’t aid for vaccine trials – The US drugmaker had received $483m in April for early-stage human trials for a coronavirus treatment = total $955 million contact from BARDA. – READ, CREDIT, Yahoo – READ, Market Watch – READ
- Remember this company at this time has no track record. They have NEVER successfully brought a product to market yet receive nearly 1 billion dollars in tax payer funds.
July 26, 2020 – PRESS RELEASE: Moderna Announces Expansion of BARDA Agreement to Support Larger Phase 3 Program for Vaccine (mRNA-1273) Against COVID-19 – READ
July 14, 2020 – PRESS RELEASE: Moderna to Join the NASDAQ-100 Index Beginning July 20, 2020 – Moderna’s common stock began trading on The Nasdaq Global Select Market on December 7, 2018 – READ
July 14, 2020 – PRESS RELEASE: Moderna Announces Publication in The New England Journal of Medicine of Interim Results From Phase 1 Study of Its mRNA Vaccine Against COVID-19 (mRNA-1273) – READ
NEJM: An mRNA Vaccine against SARS-CoV-2 — Preliminary Report – Jackson et al – Moderna study group – ARCHIVE, Peer reviewed Nov 12, 2020 – READ
- Interim analysis of original cohorts of Phase 1 study evaluated two-dose vaccination schedule of mRNA-1273 across three dose levels (25, 100, 250 µg) in 45 healthy adults ages 18-55 years; results reaffirm and expand upon positive interim data announced on May 18, 2020
- “The mRNA-1273 vaccine induced anti–SARS-CoV-2 immune responses in all participants, and no trial-limiting safety concerns were identified.”
- Even though participants experienced a severe reaction – READ
July 14, 2020 – ClinicalTrials.gov Identifier NCT04470427: A Study to Evaluate Efficacy, Safety, and Immunogenicity of mRNA-1273 Vaccine in Adults Aged 18 Years and Older to Prevent COVID-19 ( Moderna, BARDA, NIH/NIAID) Phase 3 COVE clinical trial – READ, ARCHIVE, PRESS, NIH- PRESS, Submitted July 11, 2020 – ARCHIVE
- Primary Purpose: “Prevention”
- Masking: Quadruple (Participant, Care Provider, Investigator, Outcomes Assessor)
- Current Primary Outcome Measures: Number of Participants with a First Occurrence of COVID-19 Starting 14 Days after Second Dose of mRNA-1273 [Time Frame: Day 29 (second dose) up to Day 759 (2 years after second dose)] – REF,
- The Highwire deep dive into the protocol – 95% effective for symptoms NOT infection – WATCH

July 9, 2020 – PRESS RELEASE: Moderna and ROVI Announce Collaboration for OUS Fill-Finish Manufacturing of Moderna’s COVID-19 Vaccine Candidate – READ
- A collaboration for large-scale, commercial fill-finish manufacturing of Moderna’s mRNA vaccine at Laboratorios Farmacéuticos Rovi (ROVI), a pan-European pharmaceutical company, facility in Madrid, Spain.
July 8, 2020 – PRESS RELEASE: Moderna Completes Enrollment of Phase 2 Study of its mRNA Vaccine Against COVID-19 (mRNA-1273) –READ
- Cohorts of younger adults (n=300) and older adults (n=300) in Phase 2 study fully enrolled
- Cohorts of older adults (ages 56-70, n=30) and elderly adults (ages 71 and above, n=30) in NIH-led Phase 1 study completed enrollment
June 30, 2020 – US SEC: Moderna, Inc. Q2 Report – PDF, Daily Clout Report 95 & 96 – READ & READ
- Page 70, FDA still considers mRNA a “gene therapy” product with not regulatory pathway established – READ, the same as in 2019 – TIMELINE. yet all global regulators allowed the product to enter the regulatory pathway classified as a “vaccine” under Emergency use legislations.
June 25, 2020 – PRESS RELEASE: Moderna and Catalent Announce Collaboration for Fill-Finish Manufacturing of Moderna’s COVID-19 Vaccine Candidate – READ
- Collaboration with Catalent Inc. biologics facility in Bloomington, Indiana for vial filling and packaging capacity to support production of an initial 100 million doses of the vaccine candidate intended to supply the U.S. market
June 11, 2020 – PRESS RELEASE: Moderna Advances Late-Stage Development of its Vaccine (mRNA-1273) Against COVID-19 – an update – READ
June 10, 2020 – Fierce Pharma: Moderna, AstraZeneca and J&J coronavirus shots rev up for NIH tests beginning in July: WSJ reports – READ
- Cronavirus vaccines are making their way through early stages of human testing, the NIH researchers are planning to run phase 3 trials of vaccines from Moderna (July), AstraZeneca (Aug) and Johnson & Johnson (Sept) in the coming months…said John Mascola. “All three of the shots are based on brand-new technologies, and they’re all reportedly among finalists in the Operation Warp Speed program.”
June 10, 2020 – Fierce Pharma: Moderna, AstraZeneca and J&J coronavirus shots rev up for NIH tests beginning in July: WSJ – READ
June 5, 2020 – Fierce Pharma: After Operation Warp Speed picks 5 finalists, experts question why some vaccines were left out – READ – New York Times: Trump Administration Selects Five Coronavirus Vaccine Candidates as Finalists – READ
- Moderna, Oxford/AstraZeneca, Johnson & Johnson, Merck and Pfizer, the first three having already received $2.2 billion in federal funding.
June 2, 2020 – PRESS RELEASE: Moderna Highlights Advances in Platform Science and Innovative Vaccine Research at Third Annual Science Day – Moderna will present new platform science and preclinical research – mRNA platform, LNP technology and Collaborating on HIV vaccine – READ
May 29, 2020 – PRESS RELEASE: Moderna Announces First Participants in Each Age Cohort Dosed in Phase 2 Study of mRNA Vaccine (mRNA-1273) Against Novel Coronavirus – expected to enrol 600 participants – READ
- “This Phase 2 study, being conducted by Moderna under its own Investigational New Drug (IND) application, will evaluate the safety, reactogenicity and immunogenicity of two vaccinations of mRNA-1273 given 28 days apart.
- The Company intends to enroll 600 healthy participants across two cohorts of adults ages 18-55 years (n=300) and older adults ages 55 years and above (n=300).
- Each participant will be assigned to receive placebo, a 50 μg or a 100 μg dose at both vaccinations.
- Participants will be followed through 12 months after the second vaccination.
- Given the 25 μg and 100 μg dose levels in the Phase 1 study showed neutralizing antibody titers at or above convalescent sera and were generally well tolerated, the Company has decided not to pursue the 250 μg dose level in the Phase 2 study.”
May 26, 2020 – Stat News: He experienced a severe reaction to Moderna’s Covid-19 vaccine candidate. He’s still a believer – – READ, CREDIT
- Trial volunteer – 29 year old Ian Haydon in Seattle – Received the high dose, 10x what others got – Twelve hours after receiving his second dose, he developed a fever of more than 103 degrees, sought medical attention, and, after being released from an urgent care facility, fainted in his home.
- 3 out of 15 trial participants had severe reactions – @34minREF
May 22, 2020 – Informed Consent Action Network (ICAN) submitted a FOIA request for “All safety and efficacy data and information regarding mRNA-1273, including from the Phase I clinical trial of this experimental vaccine conducted by the National Institute of Allergy and Infectious Diseases.” – READ, also see July 20, 2021 when judge ordered the “unredacted” version of the report.
May 22, 2020 – NPR: Fauci Voices Cautious Optimism About Moderna Vaccine, Calling Trial ‘Quite Promising’ – READ
- But the company, which is developing the vaccine in collaboration with the National Institute of Allergy and Infectious Diseases, which Fauci leads, has yet to publish detailed results from the trial, fueling concerns among researchers about how much the announcement can be trusted.
May 21 2020 – Moderna now receiving their vaccine development from NIH BARDA – Operation Warp Speed – ARCHIVE
May 19, 2020 – STAT news: Vaccine experts say Moderna didn’t produce data critical to assessing Covid-19 vaccine – READ
Phase 1 trial critique:
“While Moderna blitzed the media, it revealed very little information — and most of what it did disclose were words, not data.”…critical information was withheld
“The National Institute for Allergy and Infectious Diseases has partnered with Moderna on this vaccine. Scientists at NIAID made the vaccine’s construct, or prototype, and the agency is running the Phase 1 trial.” …”But NIAID did not put out a press release Monday and declined to provide comment on Moderna’s announcement.”
Phase 1 trial included “45 subjects (in this analysis) who received doses of 25 micrograms (two doses each), 100 micrograms (two doses each), or a 250 micrograms (one dose) developed binding antibodies.”
Only “eight volunteers — four each from the 25-microgram and 100-microgram arms — developed neutralizing antibodies.” Results from the other 37 trial participants was not reported and is “reason for caution”. Testing for neutralizing antibodies is more time-consuming than other antibody tests and must be done in a biosecurity level 3 laboratory.
Neutralizing antibody tests were done on blood drawn at 2 weeks post second dose. ““That’s very early. We don’t know if those antibodies are durable, said Anna Durbin, a vaccine researcher at Johns Hopkins University.”
“…studies have shown antibody levels among people who have recovered from the illness vary enormously; the range may be influenced by the severity of a person’s disease”. Yet Moderna said their antibody levels “were on a par with…those seen in people who have recovered from Covid-19 infection.” “…there’s no real way to know what that comparison means.”
Moderna told STAT that antibody data “will be disclosed in an eventual journal article from NIAID…”
May 18, 2020: Stansbury Research: Moderna Posts Positive Data on COVID Vaccine – READ
“This morning, Moderna (MRNA) announced positive interim trial data for its potential coronavirus vaccine. The data, from a National Institutes of Health (“NIH”) Phase I study, showed that MRNA’s vaccine candidate was “generally safe and well tolerated.” The company added that people who had received two doses of the vaccine showed the same level of “binding antibodies” as patients who have recovered from COVID-19…
An effective vaccine would go a long way towards helping the economy recover. In an interview with 60 Minutes over the weekend, Federal Reserve chair Jerome Powell said the economy may not hit a full recovery until we have a vaccine.”
May 18, 2020 – Moderna’s COVID-19 vaccine stimulates an immune response in people – An experimental vaccine may help protect against a coronavirus infection, preliminary results from people and mice suggest. – READ
- “One or two doses of an mRNA vaccine prod people’s bodies to make as many or more antibodies against the coronavirus as are made by people who have recovered from COVID-19, researchers from Moderna, Inc., announced May 18.”
- This first phase of testing was designed to determine safety, help researchers determine what dose to use, and to measure people’s immune response.
May 18, 2020 – MODERNA PRESS RELEASE: Moderna Announces Positive Interim Phase 1 Data for its mRNA Vaccine (mRNA-1273) Against Novel Coronavirus – ARCHIVE – Published by December 2020 – PAPER
“Noting 8 of the 45 patients in its Phase one study showed the same level of “binding antibodies” as patients who have recovered from COVID-19. It labeled the inoculation as “generally safe and well tolerated.”…”vaccine experts said that Moderna’s press release featured very little data” and “In addition, the blood samples were taken after two weeks, which is apparently very early in the process.” – REF
May 19, 2020 – STAT News: Vaccine experts say Moderna didn’t produce data critical to assessing Covid-19 vaccine – “While Moderna blitzed the media, it revealed very little information — and most of what it did disclose were words, not data.”…surprisingly their partner NIAID led by Anthony Fauci “did not put out a press release”! – READ, The Highwire – CREDIT
May 18, 2020 – STAT New: Early data show Moderna Covid-19 vaccine generates immune response – “In a Phase 1 trial, eight patients who received two doses of the vaccine at the lowest and middle doses tested — 25 and 100 micrograms — developed neutralizing antibodies to the virus at levels similar to people who had recovered from infection” – READ, CREDIT
May 18, 2020 – PRESS RELEASE: Moderna Announces Positive Interim Phase 1 Data for its mRNA Vaccine (mRNA-1273) Against Novel Coronavirus – ARCHIVE [science by vague press release]
- Total 45 healthy trial participants ages 18-55 – slit into 15 per group to receive either dose 25ug, 100ug, 250ug – Clinical Trial – ARCHIVE
- but only 8 produced neutralizing antibodies, it’s unknown the results from the other 37 trial participants and one had a grade 3 serious adverse reaction!
- Binding and neutralizing antibodies assessed the latter is the beneficial
May 15, 2020- Business Wire: Moderna Congratulates Dr. Moncef Slaoui [Moderna ex board member] on His Appointment to Oversee the White House’s Operation Warp Speed Initiative – READ,
3 days later Slaoui sells $12.4Million in Moderna stocks! – READ
May 15, 2020 – PRESS RELEASE: Moderna Congratulates Dr. Moncef Slaoui on His Appointment to Oversee the White House’s Operation Warp Speed Initiative – READ
- Dr. Moncef Slaoui has resigned from Moderna’s Board of Directors upon appointment of his new role to oversee the White House’s Operation Warp Speed initiative. Dr. Slaoui joined Moderna’s Board of Directors in 2017
May 12, 2020 – PRESS RELEASE: Moderna Receives FDA Fast Track Designation for mRNA Vaccine (mRNA-1273) Against Novel Coronavirus – Finalizing protocol for Phase 3 study of mRNA-1273, expected to begin in early summer of 2020 – ARCHIVE
May 1, 2020 – CNBC: Moderna could begin manufacturing unproven coronavirus vaccine in July, CEO says – a 10-year partnership with Swiss drugmaker Lonza – READ
May 1, 2020 – PRESS RELEASE: Moderna and Lonza [US & Switzerland] Announce Worldwide Strategic Collaboration to Manufacture Moderna’s Vaccine (mRNA-1273) Against Novel Coronavirus – READ
April 27, 2020 – PRESS RELEASE: Moderna Announces IND Submitted to U.S. FDA for Phase 2 Study of mRNA Vaccine (mRNA-1273) Against Novel Coronavirus – Phase 2 study to begin upon Investigational New Drug (IND) application acceptance by FDA – READ
April 16, 2020 – PRESS RELEASE: Moderna Announces Award from U.S. Government Agency BARDA for up to $483 Million to Accelerate Development of mRNA Vaccine (mRNA-1273) Against Novel Coronavirus – READ
- BARDA – ASPR Award will fund development of mRNA-1273 to FDA licensure, manufacturing scale-up
- NIH-led Phase 1 study of mRNA-1273 has completed enrollment of 3 dose cohorts (25 µg, 100 µg and 250 µg); expanding to an additional 6 cohorts of older adults and elderly adult
April 14, 2020 – PRESS RELEASE: Moderna Highlights Opportunity of mRNA Vaccines at its First Vaccines Day – READ
- “Vaccines create significant value [$$$] for healthcare systems by preventing infectious disease; despite this, the vast majority of viruses do not have commercial vaccines available, representing a large opportunity” – [an opportunity to fast track their untested, new technology mRNA platform “new class of transformative medicines”…and grandfather it in for all and every disease known to man!]
April 10, 2020- Science News: Meet Sophia Upshaw, a volunteer in a coronavirus vaccine trial – Nearly 50 people in Seattle and Atlanta have begun receiving injections of a potential vaccine – READ
April 3, 2020 – Forbes: Moderna CEO Stéphane Bancel Becomes A Billionaire As Stock Jumps On Coronavirus Vaccine News – READ, CREDIT
April 3, 2020 – PM Live: Moderna chairman, Noubar Afeyan, says COVID-19 vaccine could begin phase 2 trials in spring – READ
- “Without the usual hurdles, due in large part to the urgency of the pandemic, Moderna has been able to develop and advance mRNA-1273 at a speed which is unprecedented in this research area – vaccines usually take at least a couple of years to bring through the clinic and into the market.”
April 1, 2022 – AP: Study doesn’t prove that Moderna ‘created’ coronavirus – READ, Dr John Campbell – CREDIT
March 29, 2020 – PRESS RELEASE: Moderna Provides Update on the Impact of COVID-19 on Business Operations and Clinical Program Development – READ
March 16, 2020 – PRESS RELEASE: Moderna Announces First Participant Dosed in NIH-led Phase 1 Study of mRNA Vaccine (mRNA-1273) Against Novel Coronavirus – READ
- 63 days from sequence selection to Phase 1 study dosing
- mRNA-1273 is Moderna’s 10th infectious disease vaccine to begin a clinical trial
- mRNA-1273 is an mRNA vaccine against SARS-CoV-2 encoding for a prefusion stabilized form of the Spike (S) protein, which was selected by Moderna in collaboration with investigators from the Vaccine Research Center (VRC) at the National Institute of Allergy and Infectious Diseases (NIAID)
- Study conducted by NIH under its own Investigational New Drug (IND) application!!
- Manufacture of the first clinical batch was funded by the Coalition for Epidemic Preparedness Innovations (CEPI).
March 21, 2020 – Moderna’s promotional video: About mRNA-1273, Moderna’s Potential Vaccine Against COVID-19 – WATCH, Moderna’s timeline page source – HERE, YouTube has deleted this video for “violating” it’s terms of service! – ORIGINAL
March 17, 2020 – PM Live: First patients dosed with Moderna’s COVID-19 vaccine – mRNA-1273 – READ, OTHER
- The Phase 1 trial aims to recruit 45 healthy men and women aged between 18 and 55 and will test two doses of the vaccine given 28 days apart, with 12 months’ follow-up after the second dose.
- The study is being conducted by the US National Institutes of Health (NIH) at Kaiser Permanente Washington Health Research Institute in Seattle.
- The phase 1 trial was initiated in record time, after the vaccine candidate was fast-tracked by the US government, which allowed Moderna to run some aspects of its development, such as non-human primate challenge studies, in parallel with human trials.
March 16, 2020 – BioSpace: First Patient Dosed in Moderna’s COVID-19 Vaccine Trial – READ
- One unusual aspect of the Moderna vaccine development to date is that, unlike most vaccine, it has not been first tested in laboratory animals.
- “I don’t think proving this in an animal model is on the critical path to getting this to a clinical trial,” Tal Zaks, chief medical officer at Moderna, told STAT. He pointed out that National Institute of Health (NIH) scientists are “working on nonclinical research in parallel.”
March 16, 2020 – CNBC: First human trial for coronavirus vaccine begins Monday in the US – READ
- Moderna Chairman Noubar Afeyan is also CEO of venture capital firm Flagship Pioneering, helped co-found Cambridge, Massachusetts-based Moderna in 2010. [1, 2]
March 11, 2020 – STAT News: Researchers rush to test coronavirus vaccine in people without knowing how well it works in animals – READ
March 2, 2020 – Moderna Blog:How Moderna is Building a Digital Biotech – ARCHIVE, Whitepaper – PDF
February 25, 2020- Fierce BioTech: Moderna wins bragging rights as it kick-starts first experimental coronavirus clinical trial – READ
February 25, 2020 – BioSpace” Moderna’s Coronavirus Vaccine Ready for Clinical Trials – READ
- Cambridge, Massachusetts-based Moderna shipped its first batch of mRNA-1273 to NIAID for a planned Phase I clinical trial in the U.S….mRNA-1273 is a mRNA vaccine that encodes for a prefusion stabilized form of the Spike (S) protein. It was chosen by Moderna researchers in collaboration with scientists at the NIAID Vaccine Research Center (VRC). Funding for the manufacture of the batch came from the Coalition for Epidemic Preparedness Innovations (CEPI).
- The S protein complex is part of the virus necessary for membrane fusion and host cell infection. This particular protein complex has also been the target of vaccines against the coronaviruses that cause SARS and MERS.
- The collaboration across Moderna, with NIAID, and with CEPI has allowed us to deliver a clinical batch in 42 days from sequence identification
February 24, 2020 – PRESS RELEASE: Moderna Ships mRNA Vaccine Against Novel Coronavirus (mRNA-1273) for Phase 1 Study – mRNA-1273 delivered from Company’s cGMP facility in 42 days from sequence selection – READ, PDF
February 21, 2020: Moderna had received funding from CEPI and listed mRNA-1273 as a product canditate in Preclinical development – the intent for worldwide rights. – ARCHIVE,
February 21, 2020 – To tackle the new coronavirus, scientists are accelerating the vaccine process – Researchers are turning to nontraditional approaches to create vaccines and therapeutics – READ
February 10, 2020 – PRESS RELEASE: Moderna Announces Progress in Prophylactic Vaccines Modality with CMV Vaccine Phase 2 Study Data Now Expected in Third Quarter 2020 and Expands Investment in This Core Modality with Three New Development Candidates – READ
- “mRNA-1273 to prevent novel coronavirus (2019-nCoV) disease, in collaboration with the National Institutes of Health; physical manufacturing of first batch complete, awaiting analytical testing” Feb 7, 2020
February 10, 2020 – SEC filing – Moderna 424B5 – READ
February 7, 2020 – The first clinical batch of mRNA-1273 was completed, a total of 25 days from sequence selection to vaccine manufacture. The batch then proceeded to analytical testing for release. – ARCHIVE, PRESS
January 26, 2020: Moderna has 6 infectious disease “Prophylactic Vaccines” in their pipeline. – ARCHIVE
January 26, 2020 – Moderna PRESS RELEASE: Moderna Announces Funding Award from CEPI to Accelerate Development of Messenger RNA (mRNA) Vaccine Against Novel Coronavirus – ARCHIVE
- “Over the past four years Moderna has had six positive Phase 1 clinical readouts in its prophylactic vaccines modality and moved two additional programs into development. Moderna’s technology platform, fully integrated manufacturing site and development experience, combined with a multi-year relationship with the NIH, including exploring ways to respond to public health threats, allows for the rapid identification and advancement of a vaccine candidate against 2019-nCoV.”
- “Moderna’s commitment to global public health is aligned with CEPI’s vision of creating a world in which epidemics are no longer a threat to humanity,” said Richard Hatchett, M.D., CEO of CEPI.
- “We believe our mRNA vaccine technology offers potential advantages in the speed of development and production scalability, which positions Moderna to potentially develop a vaccine against coronavirus, 2019-nCoV,” said Stéphane Bancel, CEO of Moderna.
January 23, 2020 – PRESS RELEASE: Moderna Announces Funding Award from CEPI to Accelerate Development of Messenger RNA (mRNA) Vaccine Against Novel Coronavirus – READ
January 12, 2020 – “It took Moderna’s mRNA design team just one hour to design the mRNA that we immediately put onto our manufacturing equipment.” – TIMELINE
“By January of 2020, we had already manufactured, quality controlled and delivered to several dozen patients personalised cancer vaccines.
So we had the know-how and the capacity to manufacture vaccines quickly. Thus when the sequence of the SARS-CoV-2 viurs was posted to a public web server on January 10th 2020 we got immediately to work. “
Melissa J. Moore PhD, Chief Scientific Officer at Moderna TED Talk 2022
2019
December 2019 – Moderna has never brought a product to market!
December 2019 – LEMELSON-MIT: Kizzmekia Corbett COVID-19 Vaccine: Kizzmekia Corbett is a viral immunologist and member of the National Institutes of Health (NIH) team that created the Moderna COVID-19 vaccine which uses mRNA – READ, ARCHIVE
- Corbett and her team identified the “spike protein” in COVID-19, when the virus appeared in December 2019
October 7, 2019 -Moderna blog: Sharing Science at IDWeek 2019 – ARCHIVE
- At IDWeek 2019, we also presented Phase 1 data from mRNA-1653, our combination vaccine against human metapneumovirus (hMPV) and parainfluenza virus type 3 (PIV3), as well as preclinical data from mRNA-1893, our Zika vaccine.
September 12, 2019 – Moderna Blog: Two New Scientific Milestones Validate Moderna’s mRNA Platform – ARCHIVE
- “Moderna is pioneering mRNA therapeutics and vaccines to create a new generation of medicines to address unmet medical needs” …Moderna vaccine platform, an important area for our future growth”
- Breakthrough scientific achievement: first systemic mRNA therapeutic to show production of a secreted protein in humans” ..Additionally, these data are significant because the same lipid nanoparticle (LNP) formulation is being used in our first rare disease program…”
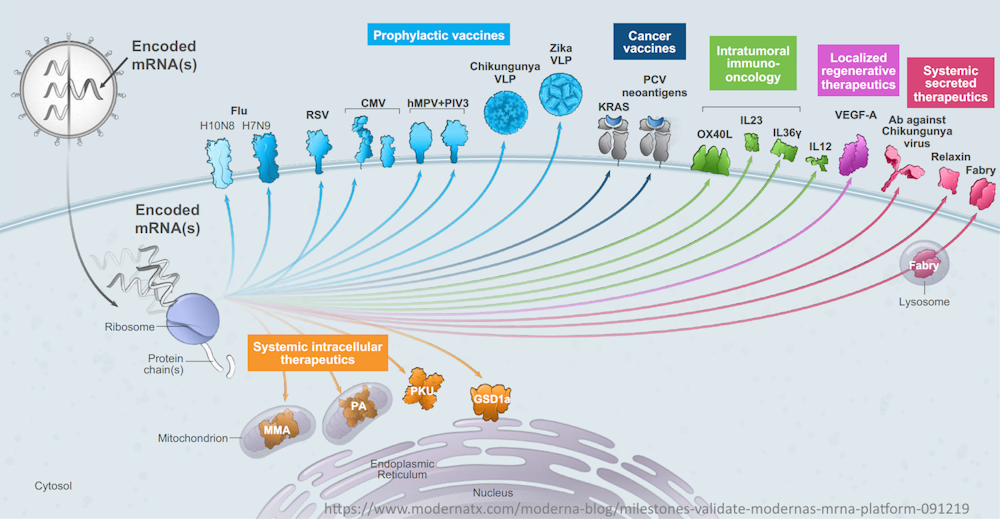
September 12, 2019 – Moderna Announces Positive Interim Results from Phase 1 Cytomegalovirus (CMV) Vaccine (mRNA-1647) Study and Progress Toward Phase 2 and Pivotal Trials – READ, CREDIT
September 12, 2019 – PRESS RELEASE: Moderna Announces Positive Phase 1 Results for the First Systemic Messenger RNA Therapeutic Encoding a Secreted Protein (mRNA-1944) – READ, Sponsored by DARPA, Agency’s ADEPT: PROTECT initiative – READ, Clinical Trial (NCT03829384) first posted February 4, 2019 – READ
- Dose study enrolled 22 healthy adults, of which 6 received placebo
- Moderna “announced positive data in the first analysis of safety and activity in its Phase 1 study evaluating escalating doses of mRNA-1944 administered via intravenous infusion in healthy adults. mRNA-1944 encodes for an antibody (CHKV-24) with activity against chikungunya virus. [note COVID-19 mRNA encodes an antigen protein]
- “These exciting data demonstrate a new way to address infectious diseases that uses mRNA to make antibodies in humans, establishing a powerful technology that could be deployable in a pandemic setting.”
July 12, 2019 – Moderna files US Patent US 10703789 (issued July 7, 2020) – MODIFIED POLYNUCLEOTIDES FOR THE PRODUCTION OF SECRETED PROTEINS – Archive PDF, CREDIT
- “A pharmaceutical composition which has a plurality of lipid nanoparticles that has a mean particle size of between 80 nm and 160 nm [nano scale] and contains a modified mRNA encoding a polypeptide . The lipid nanoparticles include a cationic lipid , a neutral lipid , a cholesterol , and a PEG lipid . The mRNA contains a 5 ‘ – cap , 5 ‘ – UTR , N1 – methyl- pseudouridine , a 3 ‘ – UTR , and a poly – A region with at least 100 nucleotides”
- Section 219 – “In another embodiment, the polymer-based self-assembled nanoparticles such as, but not limited to, microsponges, may be fully programmable nanoparticles. The geometry, size and stoichiometry of the nanoparticle may be precisely controlled to create the optimal nanoparticle for delivery of cargo such as, but not limited to, polynucleotides, primary constructs and/or mmRNA.”
July 1, 2019 -J of Inf Dis.: Protective Efficacy of Nucleic Acid Vaccines Against Transmission of Zika Virus During Pregnancy in Mice Jagger et al –READ, mRNA-1893 induced a robust antibody response and provided complete protection against transmission of Zika during pregnancy in mice – sponsored by Moderna – READ
May 10, 2019 – Journal Vaccine: mRNA vaccines against H10N8 and H7N9 influenza viruses of pandemic potential are immunogenic and well tolerated in healthy adults in phase 1 randomized clinical trials Feldmen et al – READ, Clinical Trial – READ, “against potentially pandemic avian H10N8 and H7N9 influenza viruses.“
- “Two randomized, placebo-controlled, double-blind, phase 1 clinical trials enrolled participants between December 2015 and August 2017 at single centers in Germany (H10N8) and USA (H7N9).”
- “Conclusions: The first mRNA vaccines against H10N8 and H7N9 influenza viruses were well tolerated and elicited robust humoral immune responses. ClinicalTrials.gov NCT03076385 [ARCHIVE] and NCT03345043. [ARCHIVE]” Date of clinicaltrial.gov March 2017 & Nov 2017 respectively!
May 7, 2019 – Moderna Blog: Advancing the Frontiers of Our Platform Science – ARCHIVE
- “Over the past seven years we’ve invested more than $500 million into platform research, with as much or more of those resources going to delivery science as mRNA science. In fact, we often feel like we are more of a delivery company than an mRNA company.”
- To date, our investments in delivery science have culminated in three next-generation proprietary LNP systems…” [they are still working on LNP “delivery”]
- “Today, we described our program of advancing new delivery technologies toward creating a generation of mRNA-based immune system therapeutics. This includes exciting translational work demonstrating that our novel immune nanoparticles are able to deliver mRNA to a significant proportion of T cells, Natural Killer cells, B cells and myeloid cells.We demonstrated that we can express functional proteins in vivo across multiple preclinical species, and ex vivo in human blood.” [sounds a lot like gene therapy]
February 4, 2019 – Clinical Trials gov: Safety, Tolerability, Pharmacokinetics, and Pharmacodynamics of mRNA-1944 in Healthy Adults – Phase 1 human trial of mRNA that encodes “Anti-Chikungunya Virus Monoclonal Antibody” not the antigen – DARPA funded – READ, CREDIT
2019 – Moderna: First antibody encoded by mRNA – Moderna announces dosing of the first antibody encoded by mRNA in a clinical trial – WEBSITE
2018
August 2, 2018 – BioSpace News: Moderna Executive Heads to Oncorus as New President/CEO – “There has been a number of high-level exits at Moderna over the last couple years” – READ
August 2018 – Company name changed from Moderna Therapeutics, Inc. to Moderna, Inc – REF
July 11, 2018 – BioSpace News: Moderna Provides Mid-Year Corporate Update – 21 mRNA development candidates in its pipeline with 10 in clinical development, including one program in Phase 2 and nine programs in Phase 1 – READ
March 21, 2018 – Moderna Tx Inc files Patent US10898574b2: Delivery and formulation of engineered nucleic acids – READ, Jessica Rose – harms were predicted – CREDIT
- “For example, introduced DNA can integrate into host cell genomic DNA at some frequency, resulting in alterations and/or damage to the host cell genomic DNA.”
2018 – Flagship Pioneers – Messenger RNA Timeline – ARCHIVE
- Two overarching factors have hindered the clinical progression of mRNA therapeutics:
- 1) first, the instability and immunogenicity of mRNA produced outside cells, and
- 2) second, the lack of sufficiently effective and selective delivery systems to drive protein expression in diseased cells.
Notes
- “Tackling mRNA stability, immunogenicity, and delivery, the Moderna team first found that one of mRNA’s four chemical building blocks—called uridine—can be replaced with a slightly modified nucleoside called pseudouridine. Remarkably, your immune system can’t identify mRNAs containing this modification, but your cells’ natural protein production machinery can.
- The result is that your cells recognize these synthetic mRNAs as if your body had produced them, so they can persistently generate therapeutic proteins without activating your immune system. Moderna has gone on to expand the scope of nucleoside modifications and identify effective vehicles for delivering therapeutic quantities of mRNA to diseased cells.
- The transient and programmable nature of mRNA confers broader therapeutic utility than nearly any other class of known drugs…e by simply swapping out one therapeutic gene for another.”

February 2, 2018 – BioSpace News: Mysterious Biotech Unicorn Moderna Nabs Another $500M, Is Now Worth $7B – READ
January 12, 2018 – Nature Reviews Drug Discovery: mRNA vaccines — a new era in vaccinology by Drew Weissman et al – READ Recent discoveries!!
January 3, 2018 – BioSpace News: Ex-aTyr Pharma CEO Takes Gig at Mysterious Moderna – “John Mendlein, Ph.D., a current board member and the former CEO of multiple biotechnology companies, has joined Moderna as President, Corporate and Product Strategy.” – READ
January 2, 2018 – BioSpace News: Moderna to Provide Corporate and Pipeline Updates and Outline Strategic Priorities at 2018 J.P. Morgan Healthcare Conference – READ
- Moderna aim to “create a new generation of transformative medicines for high unmet medical needs in patients – an entirely new class of medicines that directs the body’s cells to produce intracellular or secreted proteins”
2017
December 9, 2017 – TEDx Beacon Street: The disease-eradicating potential of gene editing by Tal Zaks the Chief Medical Officer of Moderna Therapeutics. – WATCH, BARCKUP, READ, MORE
“hacking the software of life” using an “operating system“
Tal Zacks
November 16, 2017 – BioSpace News: With Merck Footing the $200M Bill, Moderna Launches mRNA Cancer Vaccine Clinical Trial – dosed its first patient in a Phase I trial of mRNA-4157, a messenger RNA (mRNA)-based personalized cancer vaccine – READ
- “Moderna focuses on mRNA technology, which seems like a great idea in theory but has been extremely difficult in practice”
November 15, 2017 – MODERNA press release: Moderna Announces First-in-Human Dosing for Phase 1 Study (KEYNOTE-603) of mRNA-4157, a Personalized Cancer Vaccine, for the Treatment of Solid Tumors – READ, WATCH, TIMELINE
- Novel cancer vaccine encodes 20 neoepitopes on a single mRNA molecule to elicit a completely individualized immune response
- Moderna with mRNA-4157 in combination with KEYTRUDA® (pembrolizumab) by Merck
- [curious Paper referencing KEYNOTE-603 trial retracted – READ]
- October 18, 2017 -ClinicalTrials.gov: Safety, Tolerability, and Immunogenicity of mRNA-4157 Alone in Participants With Resected Solid Tumors and in Combination With Pembrolizumab in Participants With Unresectable Solid Tumors (KEYNOTE-603) – READ
September 2017 – Moderna: The Science of RNA – ARCHIVE
- “Moderna’s mRNA medicines aren’t small molecules, like traditional pharmaceuticals. And they aren’t biologics (recombinant proteins and monoclonal antibodies) – which were the genesis of the biotech industry.[Which Vaccines fall under in a regulatory system]. Instead, our mRNA medicines are sets of instructions. And these instructions direct cells in the body to make proteins to prevent or fight disease.”
- Describing mRNA’s role in protein synthesis & How we use mRNA as a drug
August 3, 2017 – BioSpace News: Ex-Novartis AG Exec to Lead Moderna’s Manufacturing and Operations Scale-Up – Juan Andres Joins Moderna As Senior Vice President Of Late Stage Technical Development And Manufacturing – READ
June 26, 2017 – Moderna forms a “Patent Sublicense Agreement” with CellScript, LLC – [the company that owns the sublincenc to mRNA pseudouradine components]. – READ, CONTEXT [See Nov 1, 2011 – HERE]
- “mRNA RiboTherapeutics, Inc has an exclusive license from the Trustees of the University of Pennsylvania…relating to use of modified ribonucleic acid (RNA) technology which was developed by Drs. Drew Weissman and Katalin Kariko of Penn’s Perelman School of Medicine…”
- $22 million due to CellScript by 2019.
June 2017 – Molecular Therapy: Preclinical and Clinical Demonstration of Immunogenicity by mRNA Vaccines against H10N8 and H7N9 Influenza Viruses by Bahl et al (Moderna) – READ, READ, ClinicalTrials.gov (NCT03076385) conducted in Germany – HERE, ARCHIVE
- First in-human trial with mRNA influenza vaccine
- Table 1 – Biodistribution most at injection site and lymph nodes [This info was used to justify COVID-19 vaccine stays in the arm – ARTICLE], but take a closer look at the table – it goes everywhere – are these small detectable amounts bio-active?
- It is unclear whether “placebo” was saline placebo
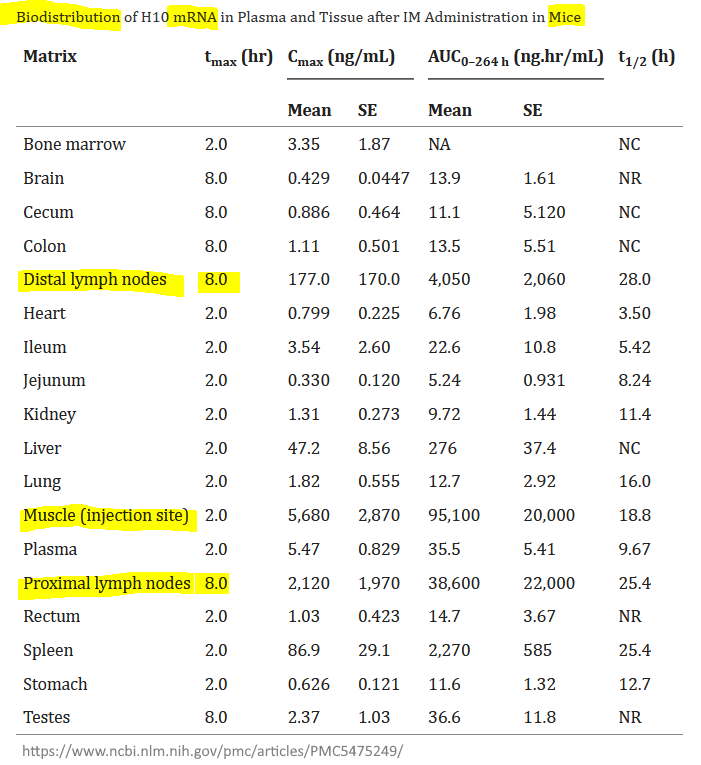
May 3, 2017 -PRESS RELEASE: Melissa J. Moore, Ph.D., Chief Scientific Officer, mRNA Research Platform at Moderna, Elected to National Academy of Sciences – READ, NAS – TIMELINE, September 13, 2016 she becomes Moderna CSO – READ
May 1, 2017 – BioSpace News: Biotech Unicorn Moderna’s Vaccine Performs Well in First Human Trial – published interim data from a Phase I study of its messenger RNA (mRNA) therapy for avian H10N8 influenza – READ
April 27, 2017 – News Wire Press Relsase: Moderna Announces Positive Interim Phase 1 Clinical Data Demonstrating First mRNA Vaccine Candidate, mRNA-1440, Induces High Levels of Immunogenicity – READ
April 27, 2017 – Molecular Therapy: Preclinical and Clinical Demonstration of Immunogenicity by mRNA Vaccines against H10N8 and H7N9 Influenza Viruses by Bahl et al – READ, funded by Moderna Therapeutics,
First in-human mRNA study – TIMELINE
- The paper states mRNA vaccines “offer advantages in speed, precision, adaptability of antigen design and production control that cannot be replicated with conventional platforms,”
- Jan. 2017 Dr Fauci warned there would be a “surprise outbreak” – TIMELINE
- Sept. 2017 – CEPI pushed the new vaccine platform – TIMELINE
- Dec 14,2017 – Fauci sets stage for “pandemic prototype vaccine” – TIMELINE
- Dec. 19, 2017 – NIH lifts ban on Gain of Function – TIMELINE
April 27, 2017 – Flagship Pioneering: Moderna Announces Positive Interim Phase 1 Clinical Data Demonstrating First mRNA Vaccine Candidate, mRNA-1440, Induces High Levels of Immunogenicity – READ, ARCHIVE, WATCH, TIMELINE
- “The majority of adverse events (AEs) were mild (107/163 events; 66%) or moderate (52/163 events; 32%), using the FDA Center for Biologics Evaluation and Research (CBER) AE severity scale” – 159/163 experienced an adverse event (AE).
- Two Influenza A strains with pandemic potential were chosen as first development candidates (CDs) in order to rapidly assess the safety and efficacy of its mRNA platform in humans
- mRNA-1440 – Influenza A Virus Subtype H10N8 Vaccine
- mRNA-1851 – Influenza A Virus Subtype H7N9 Vaccine (with pandemic potential)
- “When used as a drug, mRNA can direct cells to produce
- therapeutic proteins (mRNA therapeutics) to fight disease or
- antigenic proteins (mRNA vaccines) to prevent disease.”!
March 10, 2017 – ClinicalTrials.gov: Safety, Tolerability, and Immunogenicity of VAL-506440 in Healthy Adult Subjects – READ, ARCHIVE, TIMELINE (Influenza investigational clinical trial) May 10, 2019 –PAPER
- “A Phase 1, First-in-Human, Randomized, Double-Blind, Placebo-Controlled, Dose-Ranging Study to Evaluate the Safety and Immunogenicity of H10N8 Antigen mRNA in Healthy Adult Subjects”
- SARS-CoV-2 virus allegedly shares 19 nucleotides in the same sequence as with Moderna’s patented genome! – what are the chances of that?
- Curiously the PAPER states trial started in Dec 2015, yet the archive Clinical trial of NCT03076385 is dated March 10, 2017 – ARCHIVE – so when was the first human dosing and did they change the dates?
March 7, 2017 – Patent number US9587003B2: MODIFIED POLYNUCLEOTIDES FOR THE PRODUCTION OF ONCOLOGY RELATED PROTEINS AND PEPTIDES – by Stephan Bancel et al Moderna Therapeutics Inc. -(filed Feb 4, 2016) READ, PDF, Dr John Campbell – CREDIT,
- Moderna Patented Key Spike Protein Sequence in 2016 – A” study published February 21, 2022, in Frontiers in Virology claims to have discovered that a sequence of the virus’ spike protein is a 100% match to a modified messenger RNA (mmRNA) sequence patented3 by Moderna — in 2016.”- READ
- Study (2022) Finds Genetic Material Patented by Moderna Pre-Pandemic in COVID Spike Protein – Furin clevage site – READ
- The study identified “19-nucleotide-long RNA sequence found in the virus’s furin cleavage site matching a 19-nucleotide sequence in a genetic segment owned and patented by Moderna in February 2016“
January 12, 2017 – DARPA Tweet: Moderna has begun Phase I trials of DARPA-funded mRNA-1325 #Zika vaccine & cleared to begin trials of mRNA-1388 #Chikungunya #vaccine – TWEET, DARPA preparing for Pandemic X in 60 days or less – CREDIT
January 9, 2017 – 35th Annual J.P. Morgan Banking Healthcare Conference – Moderna Corporate Presentation by Stéphane Bancel, Chief Executive Officer, Moderna – WATCH , SOURCE
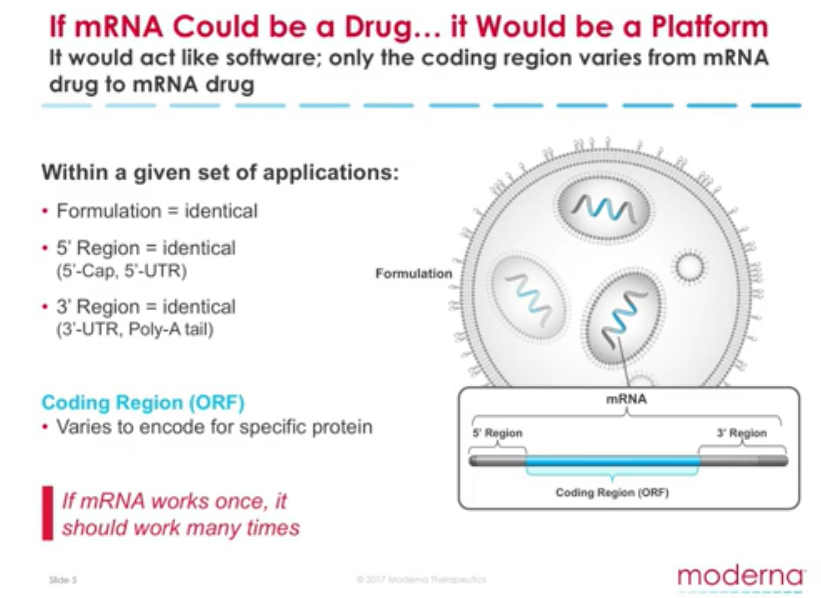
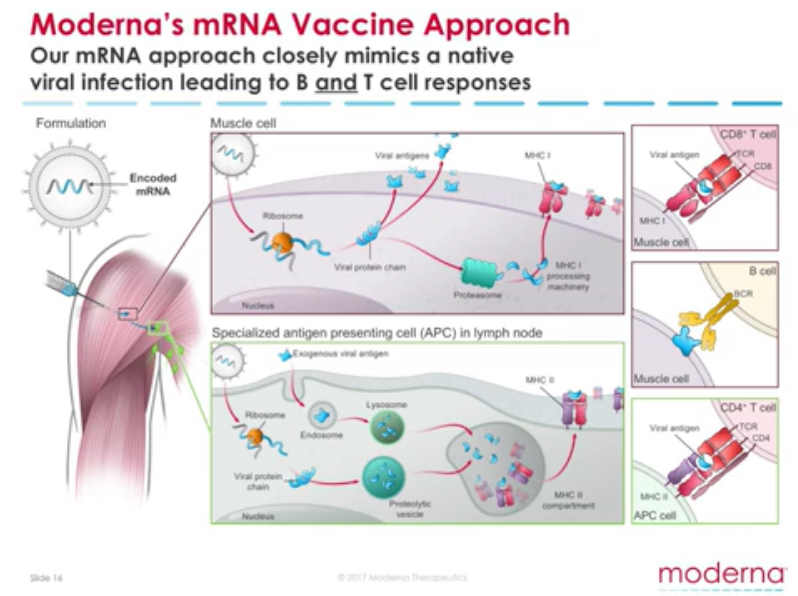
- The objective is “to make one vaccine, for one human being at a time” – EXCERPT
- “some of the mRNA gets into the muscle…” – EXCERPT, so where does the rest go?
January 4, 2017 – PRESS RELEASE – Moderna Joins the Human Vaccines Project to Help Advance Fundamental Understanding of the Immune System – READ, READ
- Human Vaccines Project is a non-profit public-private partnership that started in 2016 – with the mission to accelerate the development of vaccines and immunotherapies – “Pioneering a New Era of Human Health” – ARCHIVE, TIMELINE
2017 – First human dose of multivalent vaccine – “Moderna initiates first-in-human dosing for mRNA-1653, a combination vaccine with the potential to protect against more than one disease – human metapneumovirus (hMPV virus) and parainfluenza virus.” – WEBSITE
2016
2010-2016 – Moderna Press Release and Media Coverate – ARCHIVE
September 13, 2016 -PRESS: Moderna Appoints Melissa J. Moore, Ph.D., as Chief Scientific Officer of Moderna’s mRNA Research Platform, after serving as a member of the company’s Scientific Advisory Board – READ
- Her role will be to oversee Moderna’s efforts to advance messenger RNA to clinical pipeline – READ
- Moore is Co-Director of the RNA Therapeutics Institute (RTI) at University of Massachusetts Medical School (UMMS) in June 2009 – TIMELINE
- In May 2017 Moore is elected to the prestigious and influential, yet supposedly independent government advisory group the National Academy of Sciences – READ
September 21, 2016 – PRESS: Moderna to Build State-of-the-Art Fully Integrated GMP mRNA Clinical Manufacturing Facility in Norwood, Mass – READ, Fact Sheet – READ
- Moderna’s 200,000 square foot Norwood facility will enable the manufacture, quality, control and supply of clinical grade mRNA therapies and vaccines for Good Laboratory Practices (GLP) toxicology studies as well as Phase 1 and Phase 2 clinical studies., to open early 2018
- In addition, Moderna has amassed deep institutional expertise in the U.S. and global regulatory landscape…helping to advance programs through regulatory processes.
July 22, 2016 – Moderna was “incorporated under the laws of the State of Delaware” from SEC filing – REF
- “We are the successor in interest to Moderna LLC, a limited liability company formed under the laws of the State of Delaware in 2013. Moderna LLC was the successor in interest to Moderna Therapeutics, Inc., a Delaware corporation incorporated in 2009 as Newco LS18, Inc. by Flagship Pioneering“
January 12, 2016 – PRESS RELEASE: Moderna partnership with Bill & Melinda Gates Foundation to support development of HIV mRNA antibody program..Global health partnership may include additional mRNA-based development projects for various infectious diseases with funding up to a total of $100 Million – READ
January 2016 – Moderna/Valera: Bill & Melinda Gates Foundation — Advancing an mRNA-based antibody combination to help prevent HIV infection – committed $20M with $100M upside, with an obligation to grant to the Bill &Melinda Gates Foundation certain non-exclusive licenses – ARCHIVE, READ
2015
2015 – Moderna Press Releases 2010-2015 – ARCHIVES
- Moderna: Our Core “Expression” Platform: messenger RNA Therapeutics™ video – ARCHIVE
December 2015 – Drug Modalities: Moderna is developing mRNA-based vaccines that enable the body to produce and present immunogenic proteins to the immune system – ARCHIVE


January 8, 2015 – PRESS: Moderna Launches New Venture Valera LLC for Infectious Diseases – advance mRNA vaccines and mRNA-based passive immunity therapies – READ, their second VENTURE
Valera, a venture company formed, funded and wholly-owned by Moderna Therapeutics, is focused exclusively on the advancement of vaccines and therapeutics for the prevention and treatment of viral, bacterial and parasitic infectious diseases. Valera is leveraging Moderna’s messenger RNA Therapeutics™ platform, an entirely new in vivo drug technology that produces human proteins, antibodies and entirely novel protein constructs inside patient cells, which are in turn secreted or active intracellularly. – ARCHIVE
2015 – Moderna: “Moderna initiates first-in-human dose of an mRNA vaccine (mRNA-1440), an H10N8 flu vaccine candidate” – WEBSITE
2015 – Moderna – DARPA – Deploying mRNA Therapeutics™ for biodefense – ARCHIVE
- “The Defense Advanced Research Projects Agency (DARPA) has awarded Moderna up to $25 million to research and develop its messenger RNA (mRNA) Therapeutics™ platform as a rapid and reliable way to make antibody-producing drugs to protect against a wide range of known and unknown emerging infectious diseases and engineered biological threats.”
- “mRNA Therapeutics™ can enable the production of antibodies within the body without the need to expose people to a weakened or inactivated virus or pathogen, as is the case with the vaccine approaches currently being tested. “
2014
November 11-12, 2014 – 2nd International mRNA Health Conference, Cambridge, MA, USA – Organised & hosted by Moderna, CureVac and BioNTech – READ and sponsored by…
- First mRNA conference October 23-24 2013 – READ
October 1, 2014 – Journal of Young Investigators: Interview with Dr. Derrick Rossi of Moderna Therapeutics – READ
- Rossi’s company was recently recognized June 2014 as one of the top 10 most disruptive companies in America by CNBC, With Founders: Noubar Afeyan, Robert Langer, Kenneth Chien, and Derrick Rossi and CEO: Stéphane Bancel – READ
June 17, 2014 -List 2014 Disruptor companies #8 Moderna Therapeutics – Reprogramming cells to fight disease With Founders: Noubar Afeyan, Robert Langer, Kenneth Chien, and Derrick Rossi and CEO: Stéphane Bancel – READ
2014 – Moderna Expands: New headquarters and labs open in Cambridge, Massachusetts. – WEBSITE
2013
December 27, 2013 – TEDx Beacon Street: What if mRNA could be a drug? | Stephane Bancel – WATCH, BACKUP, CREDIT
- mRNA creates an immune response and is unstable – until now! – EXCERPT
October 2, 2013 – Fierce Biotech: Moderna lands $25M grant to develop its RNA platform against infectious diseases, bioterror – READ
October 2, 2013 – Moderna Press Release: DARPA Awards Moderna Therapeutics A Grant For Up To $25 Million To Develop Messenger RNA Therapeutics™ – READ, READ, ARCHIVE, Deploying mRNA Therapeutics™ for biodefense – READ
- Defense Advanced Research Projects Agency (DARPA) has awarded the company up to $25 million to research and develop its messenger RNA therapeutics™ platform as a rapid and reliable way to make antibody-producing drugs to protect against a wide range of known and unknown emerging infectious diseases and engineered biological threats.
- This grant is part of a DARPA program called ADEPT: PROTECT (Autonomous Diagnostics to Enable Prevention and Therapeutics: Prophylactic Options to Environmental and Contagious) – funding, CONTEXT
- “This $24.6 million grant could support research for up to 5 years to advance promising antibody-producing drug candidates into preclinical testing and human clinical trials.
March 22, 2013 – Harvard: Company that began with an HSCI seed grant announces $240M investment by Astra Zeneca in Moderna Therapeutics, a start-up company founded by HSCI Principal Faculty Derrick Rossi – READ, ARCHIVE, reports NY Times: AstraZeneca Makes a Bet on an Untested Technique – READ
March 2013 – Moderna received a $700,000 “seedling” grant from DARPA in March 2013 to begin work on their messenger RNA therapy platform – REF, REF
March 2013 – Moderna Therapeutics (Tx) – Delivering the Next Wave of Innovation to Treat Serious Illness – ARCHIVE
- we are in a unique position of pioneering an entirely new field with a dominant intellectual property estate, having “filed 144 patent applications reciting 6,910 claims directed to the company’s technology platform and drug discovery and development programs, including novel chemical modifications, RNA engineering, formulation, composition of matter, route of administration, and dosing paradigms.” – Strong Foundations READ
2012
December 6, 2012 – PRESS RELEASE: Moderna Announces $40 Million In Financing To Advance Development Of New Biotherapeutic Modality: Messenger RNA Therapeutics™ – READ, PDF
- Messenger RNA Therapeutics™ is “a novel biotherapeutic modality with the unprecedented capability of stimulating the body’s natural ability to produce therapeutic proteins. Funding led by Flagship Ventures and private investors”
- Moderna was founded within Flagship VentureLabs™, an innovation foundry dedicated to institutional entrepreneuring… an incubator program associated with Cambridge-based Flagship Ventures, which invests in early-stage biotech companies. [now Flagship Pioneering]
- Over the past 18 months, Moderna has conducted proof-of-concept studies in preclinical models, including non-human primates…
December 6, 2012 – The Boston Globe: New Cambridge biotech comes out of stealth mode – READ
- Moderna was founded 18 months ago… The initial $40 million in funding from venture capital firm Flagship Ventures and private investors. “Moderna Therapeutics is aiming to create drugs that stimulate production of proteins to treat cancer, genetic diseases, hemophilia, diabetes, and other conditions.”
December 2012 – Moderna Website ARCHIVE, About – READ
- “Moderna is pioneering messenger RNA therapeutics™, an entirely new in vivo drug modality that produces human proteins or antibodies inside patient cells, which are in turn active intracellularly or secreted.” Human Protein Therapeutics 3.0
- “Moderna has developed a broad intellectual property estate including 144 patent applications with 6,910 claims ranging from novel nucleotide chemistries to specific drug compositions.” initially focused on “rare diseases and oncology”
- “Based in Cambridge, Massachusetts, Moderna is privately held and was founded in 2010 by Flagship VentureLabs in association with leading scientists from Boston Children’s Hospital and Massachusetts Institute of Technology.”
2011
October 2011 – Moderna’s operations start – Moderna begins research into the production of mRNA medicines. WEBSITE, Leadership Team at Moderna – ARCHIVE
- Noubar of Flagship Pioneering recruits Stéphane Bancel who joins as Senior Partner and Newco LS18, Inc board of directors in March 2011, becoming Moderna CEO in October 2011 – REF
- According to Flaghship Pioneering’s Archived webpage – Stéphane Bancel joined Flagship Pioneering as a senior partner in 2013 – When he “is president and founding chief executive officer of Moderna Therapeutics, a Flagship VentureLabs company…Prior to his time at bioMérieux, Stéphane was managing director of Eli Lilly in Belgium and executive director of global manufacturing strategy and supply chain at Eli Lilly in Indianapolis.
- Stéphane was elected a 2009 Young Global Leader by the World Economic Forum
August 4, 2011 – Xconomy: Stéphane Bancel, Former bioMérieux CEO, Talks Future of Startups, Diagnostics, Pharma – Bancel is also involved with Cambridge-based ModeRNA, a stealthy therapeutics startup backed by Flagship Ventures – READ
2011 – Flagship VentureLabs – First refered to the startup company as ModeRNA – ARCHIVE, Pioneering process – READ
2010
December 9, 2010 – PRESS RELEASE: Flagship VentureLabs Company ModeRNA Therapeutics, and co-founder, Derrick Rossi, both Cited by Time Magazine for Significant Advances and Contributions in 2010 – READ
October 4, 2011 – Xconomy: ModeRNA, Stealth Startup Backed By Flagship, Unveils New Way to Make Stem Cells – READ
October 1, 2010 – Reuters: U.S. researchers make stem cells quickly from skin – Dr. Derrick Rossi of Harvard Medical School and colleagues – READ
- It takes just 3 or 4 genes to turn back the clock on skin cells or other ordinary cells, and make them behave like stem cells. But most ways of doing this involve using a virus to carry the new genes into the cell, or DNA, and these techniques can lead to other problems, including tumors….the new method uses RNA.
September 30, 2010: PRESS RELEASE: Flagship VentureLabs company, ModeRNA Therapeutics, Announces Safe and Efficient Method for Creating and Differentiating Human Pluripotent Stem Cells – READ
September 30, 2010 – Harvard Gazette: Breakthrough in cell reprogramming – Harvard Stem Cell Institute (HSCI) researches progress toward producing cells safe to use as disease treatments – READ, Sciene Mag – READ, White Coat Notes – READ
- Derek Rossi profile at Harvard – ARCHIVE, 2011 – ARCHIVE
- a startup company called ModeRNA Therapeutics has been formed
September 30, 2010 – Cell Journal: Highly Efficient Reprogramming to Pluripotency and Directed Differentiation of Human Cells with Synthetic Modified mRNA by Derek Rossi et al – READ, MIT – READ
- “The researchers created synthetic RNA molecules, adding chemical modifications that enabled the molecules to escape immune attack when introduced into a cell”
- Derek J Rossi recently founded a company, ModeRNA Therapeutics, dedicated to the clinical translation of this technology. [Unsure if anything to do with modeRNA for 3D modeling of RNA structures, which mentions Moderna – HERE]
2010 – Moderna is incorporated (founded) -“Moderna’s name combines the words “modified” and “RNA“, which happens to contain the word “modern.” – WEBSITE
- “Moderna is creating potential mRNA medicines for a wide range of diseases and conditions which currently include infectious diseases, oncology, rare genetic diseases, and cardiovascular diseases. Moderna is developing these potential medicines independently and through strategic alliances with collaborators, including AstraZeneca, Merck, and Vertex Pharmaceuticals, as well as the Defense Advanced Research Projects Agency (DARPA), the Biomedical Advanced Research and Development Authority (BARDA), and the Bill & Melinda Gates Foundation” – REF
Spring 2010 – Flagship Pioneering invested in Moderna – HERE, Moderna/RNA Timeline at Flagship – ARCHIVE
- “Noubar Afeyan meets with Bob Langer and Derrick Rossi to review recent data on the use of chemically modified mRNA to reprogram adult human fibroblast cells into induced pluripotent stem cells following protocol of Prof. Yamanaka”
2010 – Flagship Ventures | Flagship VentureLabs co-founded ModeRNA Therapeutics – ARCHIVE, ARCHIVE, About Flagship Ventures – ARCHIVE
- “Flagship VentureLabs co-founded ModeRNA Therapeutics with world-leading academic researchers Robert Langer and Kenneth Chien to commercialize a new class of RNA-based drugs. ModeRNA’s technology platform, invented by Assistant Professor Derrick Rossi of Harvard, also a company co-founder, makes it possible to direct cells to express proteins without changing their genome or creating long term safety concerns. ModeRNA intends to use its technology to create drugs that target a range of diseases with high unmet need.” with Flagship Partners: Doug Cole, Noubar Afeyan
- “Noubar envisions a different use of engineered mRNA as a new kind of medicine and launches within Flagship’s VentureLabs group a month-long exploration which eventually leads to Newco LS18, Inc., this will eventually become Moderna Therapeutics – REF,
- Noubar recruits Stéphane Bancel who joins Flagship as Senior Partner and LS18 board of directors in March 2011, becoming Moderna CEO in October 2011 – REF
May 28, 2010 – Harvard HSCI: Disease Foundations Team Up with HSCI to Hasten Cures – READ
- HSCI faculty member Derrick Rossi, PhD, whose lab will contribute to generating the immune system in the humanized mouse model. Other scientists participating in this effort are Dale Greiner, PhD, University of Massachusetts Medical School…
2009
June 2009 – RNA Therapeutics Institute is formed at University of Massachusetts Medical School, where future Moderna Chief Scientific Officer Prof Melissa J. Moore, PhD is a co-director – ARCHIVE, TIMELINE
2009 – Modena began it’s company journey in 2009 as Newco LS18, Inc. by Flagship Pioneering accoring to SEC filing – REF, Flagship Pioneers many companies – LIST

2009 – Stéphane Bancel was elected a 2009 Young Global Leader by the World Economic Forum, then joined Flagship Pioneering in 2013, and was president and founding chief executive officer of Moderna Therapeutics, a Flagship VentureLabs company, he would go on to become Moderna’s CEO and become a billionaire! – REF
2004
To Be confirmed:
August 27, 2004 – NSF Launches Chemical Bonding Centers Program – 1) Darwinian Chemical Systems – READ
- “With this Chemical Bonding Center (CBC) Phase I, Step II award, the Division of Chemistry and the Office of Multidisciplinary Activities of the Mathematical and Physical Sciences Directorate jointly support the research of Jack W. Szostak, of Massachusetts General Hospital, Steven A. Benner, of the University of Florida, and Gerald F. Joyce, of the Scripps Research Institute. This CBC will pursue the long-term goal of synthesizing artificial chemical systems that will exhibit Darwinian evolution. In phase I of the project, a combination of molecular design and laboratory selection will be used to generate RNA-like structures that undergo self-reproduction with heritable mutation. – REF,
- Jack Szostak received Nobel Prize in 2009 – READ, In 2013 he was at Harvard – ARCHIVE
August 12, 2004 – US National Science Foundation: Award Abstract # 0434507- Darwinian Chemical Systems – READ
- Moderna is said to be founded on back of a 10 year National Science Foundation grant project called Darwinian Chemical Systems [to be confirmed]