Janssen Pharmaceuticals is a wholly-owned subsidiary of Johnson & Johnson (J&J) and in 2020 they produced a COVID-19 vaccine [Ad26.COV2.S, recombinant] in partnership with Catalent. In June 2020 J&J partnered with the US NIAID/BARDA as part of Operation Warp Speed.
Janssen Pharmaceutica, Inc. was founded in 1953 by a researcher, pharmacologist and physician, Dr Paul Janssen and “backed by the significant resources of Johnson & Johnson, its parent company and the world’s most comprehensive manufacturer of health care products.”
Dr. Paul Janssen is considered a 20th century innovative and inspiring scientists as his work “was responsible for many breakthroughs in several fields of disease, including pain management, psychiatry, infectious diseases, mycology and gastroenterology.” Janssen has more than 100 patents to his name, of which 18 Janssen medicines are on the World Health Organization’s List of Essential Medicines.
In 1961, Janssen Pharmaceuticals was purchased by New Jersey-based American corporation Johnson & Johnson (J&J).
Janssen Pharmaceutica N.V. became part of the Johnson & Johnson Family of Companies in 1961. In 1985, Dr. Paul set up Xi’an Janssen Pharmaceutical Co., Ltd., the first Western pharmaceutical company in the People’s Republic of China.
Johnson & Johnson acquired the Dutch biotech company Crucell based in Leiden and placed it in their pharmaceutical division and refere to it as Janssen Vaccines. By February 2011 J&J had taken ownership of over 95% of Crucell and delisted it from the stock exchanges two months later. In 2014, the subsidiary was renamed from Crucell to Janssen Vaccines. Janssen Vaccines in Leiden, the Netherlands, developed the COVID-19 vaccines for Johnson & Johnson.
Official Pages:
- Janssen COVID-19 vaccine website – HERE, ARCHIVES, Fact sheets – HERE
- Johnson & Johnson website – HERE
- FDA official Janssen vaccine page – HERE, ARCHIVES
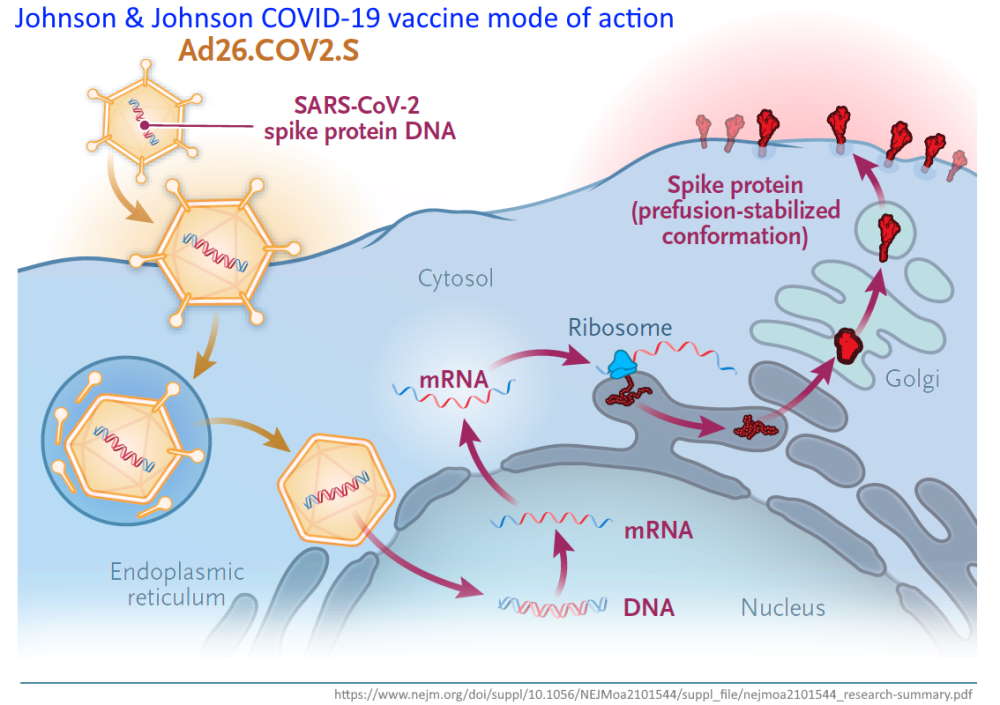
Ad26.COV2.S = Adenovirus serotype 26 (Ad26) vector-based vaccine encoding a stabilized SARS-CoV-2 spike (S)
The Janssen COVID-19 Vaccine leverages the AdVac® viral vector technology, vaccine platform, a unique and proprietary technology – READ
Adenoviruses are a group of viruses that cause the common cold – so they can enter human cells. The adenovirus vectors have been genetically modified so that it can no longer replicate in humans and cause disease.
Australia note: Because J&J and AstraZeneca use a genetically modified organism (GMO) to encapsulate their synthetic spike protein gene, the GMO component needs to be approved by the Office of Gene Technology Regulator (OGTR), but the mRNA vaccines are 100% exempt of having to be assessed because [as far as I can work out] they are not an “organism”!
Clinical Trials:
- June 18, 2020 – Clinical Trials gov: A Study of Ad26.COV2.S in Adults (COVID-19) – Phase I/II – READ, ARCHIVE
- August 10, 2020 – Clinical Trials gov: A Study of Ad26.COV2.S for the Prevention of SARS-CoV-2-Mediated COVID-19 in Adult Participants (ENSEMBLE) – PHASE III trial design Sponsor: Janssen – READ, ARCHIVE, All trial changes – ARCHIVE, History of trial changes – HERE
- January 13, 2021 – NEJM: Interim Results of a Phase 1–2a Trial of Ad26.COV2.S Covid-19 Vaccine – Sadoff et al – {J&J Clinical Trial data] – ARCHIVE, Updated May 31, 2021 – READ
The J&J vaccine was initially considered an important tool in fighting the pandemic because it required only one shot. Then the FDA issued a warning: The Janssen COVID-19 Vaccine can cause thrombosis with thrombocytopenia syndrome (TTS) which may be life-threatening. – PDF
Links in reverse chronological order
This page is continuously added to
2024
June 13, 2024 – National Pulse: Johnson & Johnson Pays $700 Million in Lawsuit Settlement for misleading customers about the safety of its talcum-based baby and body powders – READ, $149.5M in Opioid Lawsuit – READ
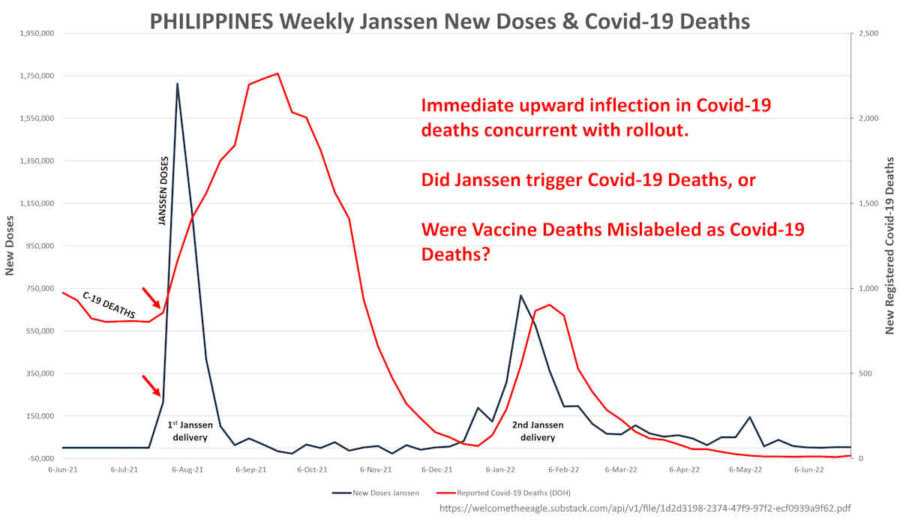
February 27, 2024 – Sally Clark Substack: The Hottest Covid-19 Batches in the Universe Were Given Exclusively to the Philippines by WHO-COVAX. Philippines Hyper-excess Deaths in Q3-2021 Might Be Explained by Janssen Hot Lots! – READ, Eagle88 Substack: Janssen (J&J) Hot Lots Announced To Filipino House of Representatives – “COVID-19” death spikes in data followed after Janssen vaccine rollout peaks – was COD mis-labelled? – CREDIT
- The Manila Times: Public Hearing of the Committee on Public Order & Dangerous Drugs joint w/ Justice and Human Rights, Chaired Congressman Dan S. Fernandez – WATCH, FB, SOURCE
- Presentation by Sally Clark: Evaluation of Philippines Population Outcomes for All-Cause Mortality and COVID-19 Deaths Relative to COVID-19 Vaccine Deployment in 2021 (J&J Hot Lot)- WATCH1, WATCH2, Stunning presentation slides – PDF
- Janssen vaccines represented 4.2% of vaccines used in Philippines but was associated with 11.1% of Serious Adverse Events (SAE) reported, the highest of any vaccine used in that country. It is still a highly under-reported pharmacovigilant system. The Philippines experienced the highest number of deaths in the world following a Janssen vaccine. Janssen rolled out July 20, 2021.
January 18, 2024 – ICAN: BREAKING: ICAN Acquires More Lot Data — This Time for the J&J COVID-19 Vaccine – exclusive lot and dose data – READ
- This “data may help make it possible to determine if certain lots were associated with unusually high numbers of adverse reactions or deaths.:”
2023
December 20, 2023 – ICAN: EXCLUSIVE: J&J (Janssen) Lot and Dose Data Release – Johnson & Johnson COVID-19 Lot Numbers and Dose Data Obtained by ICAN Through FOIA – – READ, The Eagle – CREDIT
2022
May 16, 2023 – Chief Nerd – CBS: CDC Officials Say the J&J COVID-19 Vaccine Is No Longer Available in U.S. – EXCERPT
- “All remaining doses expired last week according to the CDC”
- “Over the course of the pandemic more than 31M doses of the J&J vaccine were sent out but only 19M people got the J&J shot – that’s about 7% of Americans..leaving 12M unused…Most were told often to receive Pfizer or Moderna shots.”…PHE finished last week but people can still get free vaccinations while supplies last
May 10, 2023 – CDC Updates website: Janssen COVID-19 Vaccine is no longer available in the U.S – dump remaining stock – READ, ARCHIVE, Gateway Pundit – READ
- “Janssen COVID-19 Vaccine is no longer available in the U.S. All remaining U.S. government stock of Janssen COVID-19 Vaccine expired May 7, 2023. Dispose of any remaining Janssen COVID-19 Vaccine in accordance with local, state, and federal regulations.”
October 25, 2022 – NASDAQ: Johnson & Johnson’s blood cancer therapy gets U.S. FDA approval – READ [cancers have been on the increase since jabs rolled out]
August 25, 2022 – Clinical Trials gov: Phase 2a Trial to Evaluate Safety and Immunogenicity of COVID-19 Vaccine Strategies in HIV-infected/Uninfected Adults. (AUR1-8-341) – CEPI driven trial in South Africa, multiple vaccines including J&J, Pfizer & CovovaxTN – READ, ARCHIVE, Worldometer – IMAGE
June 8, 2022 – Clinical Trials gov: Evaluation of the Immunogenicity and Safety of COVID-19 Vaccines (Ad26.COV2.S and NVX-CoV2373) (CoviCompMali) – READ
- Trial sponsored by ANRS with CEPI et al, comparing J&J to Novavax
- Being conducted in Mali, West Africa!!! – Worldometer – HERE, Scale goes to 15- IMAGE, US scale goes to 5,000 – IMAGE
May 5, 2022 – NPR: FDA limits Johnson & Johnson’s COVID vaccine to some people due to blood clot risk – READ
May 5, 2022 – MEDIA STATEMENT: Johnson & Johnson Updates U.S. COVID-19 Vaccine Fact Sheet – to increase awareness about the risk of thrombosis with thrombocytopenia syndrome (TTS) – READ, FACT SHEET
- “The Janssen COVID‑19 Vaccine has not been approved or licensed by the U.S. Food and Drug Administration (FDA), but has been authorized by FDA through an Emergency Use Authorization (EUA)…” – WARNINGS AND PRECAUTIONS, and ADVERSE REACTIONS – – ARCHIVE
May 5, 2022 – Associate Press: FDA restricts J&J’s COVID-19 vaccine due to the ongoing risk of rare but serious blood clots. – READ
- The problem occurs in the first two weeks after vaccination. As of mid-March 2022, federal scientists had identified 60 cases of the side effect, including 9 that were fatal. That amounts to 3.23 blood clot cases per 1 million J&J shots. The problem is more common in women under 50, where the death rate was roughly 1 per million shots
- The clotting problems first came up last spring, with the J&J shot in the U.S. and with a similar vaccine made by AstraZeneca that is used in other countries. At that time, U.S. regulators decided the benefits of J&J’s one-and-done vaccine outweighed what was considered a very rare risk — as long as recipients were warned.
- COVID-19 causes deadly blood clots, too. But the vaccine-linked kind is different, believed to form because of a rogue immune reaction to the J&J and AstraZeneca vaccines because of how they’re made.
May 5, 2022 – FDA NEWS RELEASE: Coronavirus (COVID-19) Update: FDA Limits Use of Janssen COVID-19 Vaccine to Certain Individuals – READ, Fact sheet – PDF
- Restricted “to individuals 18 years of age and older for whom other authorized or approved COVID-19 vaccines are not accessible or clinically appropriate”
- Because of “risk of thrombosis with thrombocytopenia syndrome (TTS), a syndrome of rare and potentially life-threatening blood clots in combination with low levels of blood platelets” around 2 weeks following vaccination.
May 2, 2022 – Judicial Watch also received 663 pages of records from HHS regarding biodistribution studies and related data for COVID-19 vaccines, which show that Johnson & Johnson relied on studies showing that vaccine DNA particles and injected virus particles were still present in test animals months after injection. – READ
April 24, 2022 – Associate Press: FDA restricts J&J’s COVID-19 vaccine due to blood clot risk – READ
- U.S. health officials lifted an 11-day pause on COVID-19 vaccinations using Johnson & Johnson’s single-dose shot on Friday, after scientific advisers decided its benefits outweigh a rare risk of blood clots.
January 6, 2022 – PRESS RELEASE: Real World Evidence Shows Johnson & Johnson COVID-19 Vaccine Demonstrates Durable Protection Against Breakthrough Infection, Hospitalization, and Intensive Care Unit Admission in the United States – READ
2021
December 30, 2021 – PRESS RELEASE: Johnson & Johnson COVID-19 Vaccine Demonstrates 85 Percent Effectiveness against Hospitalization in South Africa when Omicron was Dominant – READ
- Separate analysis…booster generated 41-fold increase in neutralizing antibodies and a 5-fold increase in T-cells against Omicron
- …”preliminary results from the South African Phase 3b Sisonke study which showed that a homologous (same vaccine) booster shot of the Johnson & Johnson COVID-19 vaccine (Ad26.COV2.S) demonstrated 85 percent effectiveness against COVID-19-related hospitalization.”
- Conducted by the South African Medical Research Council (SAMRC) from mid-November to mid-December among healthcare workers
- “A second, separate analysis of the immune response to different vaccine regimens, conducted by Beth Israel Deaconess Medical Center (BIDMC), demonstrated that a heterologous booster (different vaccine) of the Johnson & Johnson COVID-19 vaccine in individuals who initially received the BNT162b2 mRNA vaccine [Pfizer] generated a 41-fold increase in neutralizing antibody responses by four weeks following the boost and a 5-fold increase in CD8+ T-cells to Omicron by two weeks.
- A homologous boost with BNT162b2 generated a 17-fold increase in neutralizing antibodies by four weeks following the boost and a 1.4-fold increase in CD8+ T-cells by two weeks.”
November 24, 2021 – PRESS RELEASE: Johnson & Johnson COVID-19 Vaccine Fully Approved by Health Canada to Prevent COVID-19 in Individuals 18 years and Older – READ
November 3, 2021 (First published) – European Medical Association (EMA): Jcovden (previously COVID-19 Vaccine Janssen) – COVID-19 vaccine (Ad26.COV2-S [recombinant]) – READ
October 25, 2021 – Clinical Trials gov: A Study of Ad26.COV2.S and Influenza Vaccines in Healthy Adults – READ
August 25, 2021 – NY Post: J&J says booster shot of COVID vaccine ‘increases antibodies’ – READ
August 25, 2021 – PRESS RELEASE: Johnson & Johnson Announces Data to Support Boosting its Single-Shot COVID-19 Vaccine – READ
- Six months after EUA: booster “provided rapid and robust increase in spike-binding antibodies”
- “New studies build on data demonstrating strong durability through eight months after immunization” [after vaccination not immunization!]
- Contraindications & warnings listed in presser
- COVID19
August 16, 2021 – Clinical Trials gov: A Study to Evaluate Different Dose Levels of Ad26.COV2.S in Healthy Adolescents From 12 to 17 Years Inclusive (HORIZON 2) – Phase II, 304 participants – READ, Dec 2 – ARCHIVE
August 10, 2021 – Clinical Trials gov: A Study of Ad26.COV2.S Administered as Booster Vaccination in Adults Who Have Previously Received Primary Vaccination With Ad26.COV2.S or BNT162b2 (Amplify) – READ
July 14, 2021 – PRESS RELEASE: Johnson & Johnson Single-Shot COVID-19 Vaccine Demonstrated a Durable Immune Response and Elicited Dual Mechanisms of Protection Against Delta and Other SARS-CoV-2 Variants of Concern in Data Published in New England Journal of Medicine (NEJM) – READ
- “Interim results from a Phase 1/2a sub-study published” in the NEJM [correspondence!] “demonstrated that both humoral (antibody) and cellular (T-cell) immune responses generated by the Johnson & Johnson single-shot COVID-19 vaccine were strong and stable through eight months after immunization, the length of time evaluated to date”
July 14, 2021 – NEJM Correspondence to the Editor: Durable Humoral and Cellular Immune Responses 8 Months after Ad26.COV2.S Vaccination – by J&J and Harvard Uni – READ, ARCHIVE
July 13, 2021 – CNN: FDA warns of potential rare neurological complication with Johnson & Johnson coronavirus vaccine – Guillain-Barré syndrome (GBS) – READ
July 12, 2021 – PRESS RELEASE: Johnson & Johnson Statement on COVID-19 Vaccine (7/12) – “Rare cases of the neurological disorder, Guillain-Barré syndrome have been reported following vaccination with the Janssen COVID-19 vaccine. Most occurred within 42 days after vaccination….” – READ, FDA document updated July 8, 2021 – ARCHIVE
July 1, 2021 – PRESS RELEASE: Positive New Data for Johnson & Johnson Single-Shot COVID-19 Vaccine on Activity Against Delta Variant and Long-lasting Durability of Response – READ, [unclear which “preprint”]
- Demonstrated strong neutralizing antibody activity against the Delta (B.1.617.2) variant
- Persistent immune responses through at least eight months
- Preprint submitted to bioRxiv today which contains “new analysis from blood samples obtained from a subset of participants (n=8) in the Phase 3 ENSEMBLE study”
- “In the ENSEMBLE trial, Johnson & Johnson’s single-dose COVID-19 vaccine was 85 percent effective against severe/critical disease and demonstrated protection against hospitalization and death. The vaccine was consistently effective across all regions studied globally, including in South Africa and Brazil, where there was a high prevalence of rapidly emerging Beta and Zeta (P.2) variants during the study period.”
- Vaccine available in many regions on a not-for-profit basis
July 1, 2021 – BioRxiv preprint: Ad26.COV2.S elicited neutralizing activity against Delta and other SARS-CoV-2 variants of concern by Janssen – READ
- WHO variants of concern (VOC): Alpha (B.1.1.7), Beta (B.1.351), Gamma (P.1), Delta variant (B.1.617.2), Epsilon (B.1.429) and Kappa (B.1.617.1) variants, as well as the original SARS-CoV-2 strain (WA1/2020)
- Janssen suggest immune response elicited against all these VOC
June 25, 2021 – AUSTRALIA Therapeutic Goods Administration (TGA) granted provisional approval to Janssen-Cilag Pty Ltd (known as Johnson & Johnson overseas) for its COVID-19 vaccine Janssen, making it the third COVID-19 vaccine to receive regulatory approval in Australia. BUT “The Janssen vaccine is not included in Australia’s COVID-19 vaccination program. – READ
June 1, 2021 – Clinical Trials gov: A Study to Evaluate Dose Levels of Ad26.COV2.S Administered as a Two-dose Schedule in Healthy Adults – Phase III – READ
May 20, 2021 – Clinical Trials gov: A Study of Ad26.COV2.S in Healthy Adults (COVID-19) – to assess 0.3 mL versus 0.5 mL dose – READ
April 23, 2021 – CNN: CDC, FDA lift pause on using J&J’s coronavirus vaccine, add safety warning – READ, Updated FACT SHEET – PDF
- ““We have concluded that the known and potential benefits of the Janssen COVID-19 vaccine outweigh its known and potential risks in individuals 18 years of age and older,” acting FDA Commissioner Dr. Janet Woodcock in a statement”
- ACIP committee voted 10 in favor of lifting the pause, four opposed, with one abstention.
April 23, 2021 – FDA NEWS RELEASE: FDA and CDC Lift Recommended Pause on Johnson & Johnson (Janssen) COVID-19 Vaccine Use Following Thorough Safety Review – READ, ACIP met today – READ
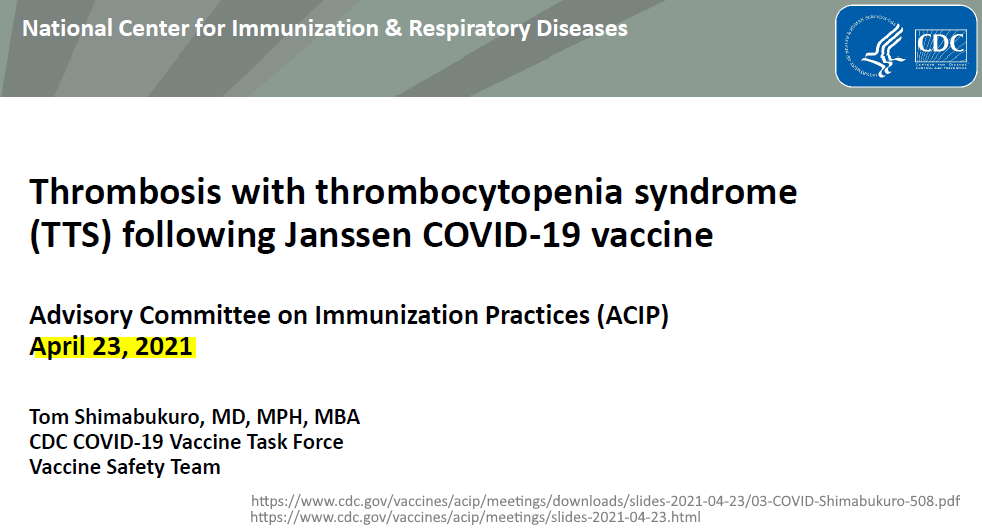
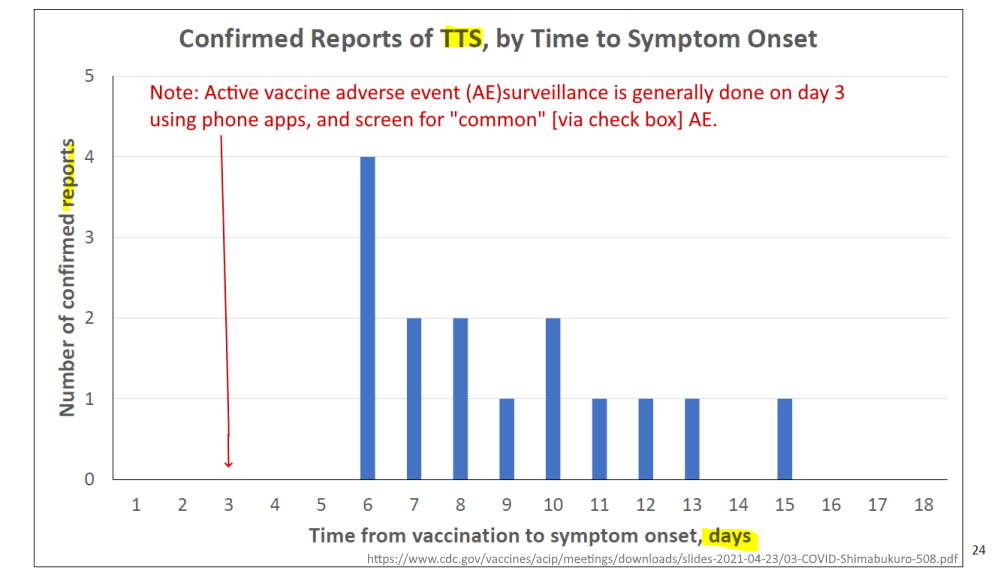
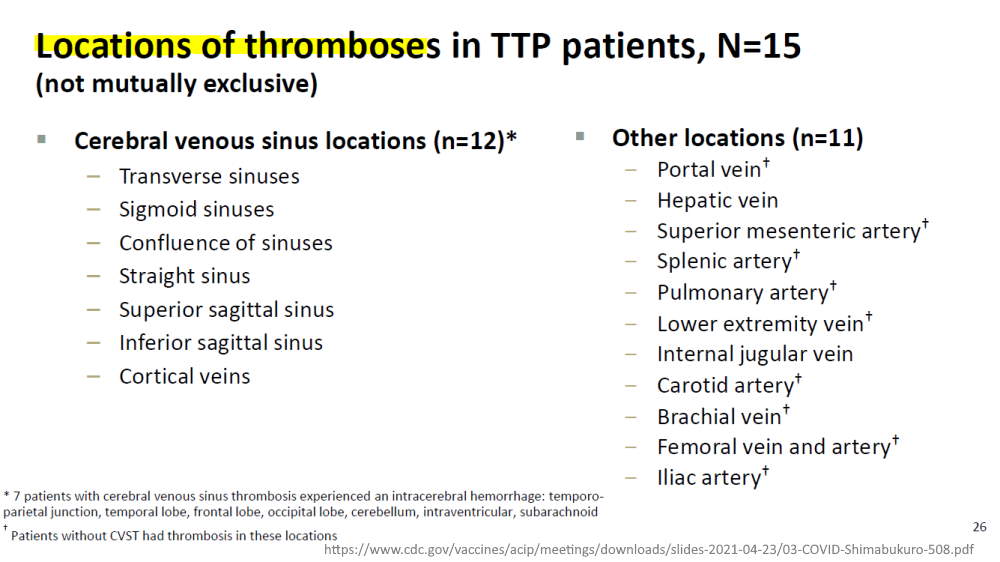
April 23, 2021 – CDC ACIP committee met to discuss Thrombosis with thrombocytopenic syndrome (TTS)- READ, MINUTES, Dr. T Shimabukuro – SLIDES,
- (slide 13) – mRNA vaccines – 10 total cases of CVST identified – without thrombocytopenia
- (slide 14) – Janssen – 6 cases VAERS, 1 case in clinical trials!!! – NEJM
- (slide 15) – Brighton Collaboration defining “standard case definition for study of new clinical syndrome X, as applied to Thrombosis with Thrombocytopenia Syndrome (TTS)” – PDF, Brighton Collaboration – ABOUT, Updated TTS definition – READ
Example Active Surveillance systems – US, V-SAFE, AusVaxSafety
April 21, 2021 – PRESS RELEASE: Johnson & Johnson Single-Shot COVID-19 Vaccine Phase 3 Data Published in New England Journal of Medicine – primary data from the Phase 3 ENSEMBLE clinical trial – READ
- Single-dose vaccine prevented hospitalization and death across all study participants, 28 days after vaccination
- Vaccine shown to be effective against severe/critical COVID-19 disease as early as seven days after vaccination, with efficacy continuing to increase eight weeks post-vaccination
- Vaccine also shown to be consistently effective against symptomatic infection , including in South Africa and Brazil where there was a high prevalence of rapidly emerging SARS-CoV-2 variants
April 21, 2021 – NEJM (fist published): Safety and Efficacy of Single-Dose Ad26.COV2.S Vaccine against Covid-19 by Sadoff et al the ENSEMBLE study group – READ, PDF
April 19, 2021 – Office of the Gene Technology Regulator (OGTR) Australia: DIR 182 – Commercial supply of a genetically modified COVID-19 vaccine – Janssen-Cilag Pty Ltd [Johnson & Johnson COVID-19 vaccine], Adenovirus, Vaccine – altered antigen expression; Vaccine – replication incompetent – READ, ARCHIVE, Date application received December 2, 2020
- Licence decision – notification – PDF
- Licence decision – Q&A – PDF
- Risk assessment and risk management plan – summary – PDF, Full – PDF
- Licence Condition – PDF
April 16, 2021 – NEJM correspondence: Thrombotic Thrombocytopenia after Ad26.COV2.S Vaccination — Response from the Manufacturer – READ, slide 14 – SOURCE
April 15, 2021 – Epoch Times: CDC Vaccine Advisory Committee Says J&J COVID-19 Vaccine to Remain on Pause – READ
April 14, 2021 – PRESS RELEASE: Johnson & Johnson Statement on CDC Advisory Committee Meeting on Company COVID-19 Vaccine – PDF, SOURCE,
- ACIP in an emergency meeting today “to consider reports of an extremely rare disorder involving blood clots in combination with low platelets observed in a small number of individuals following vaccination with the Johnson & Johnson COVID-19 vaccine.”
- Out of an abundance of caution, the CDC and FDA have recommended a pause in the use of our vaccine.
- The Company has made the decision to proactively delay the rollout of our vaccine in Europe and pause vaccinations in all Janssen COVID-19 vaccine clinical trials while we update guidance for investigators and participants.
April 14, 2021 – CDC’s ACIP EMERGENCY meeting – discuss safety of Janssen’s COVID-19 vaccine –READ, MINUTES, presentation of the 6 cases – SLIDES
- J&J vaccine will remain on “pause” in the US as the committee “needed more data to be able to vote on a recommendation, which may come in a week to 10 days.”
- Moderna has 3 CVST reports to VAERS out of 84.7 million doses
- They noted (slide 16) “Thrombosis usually does not occur in the presence of low platelets; these case presentations are atypical and consistent with cases observed after AstraZeneca COVID-19 vaccine”
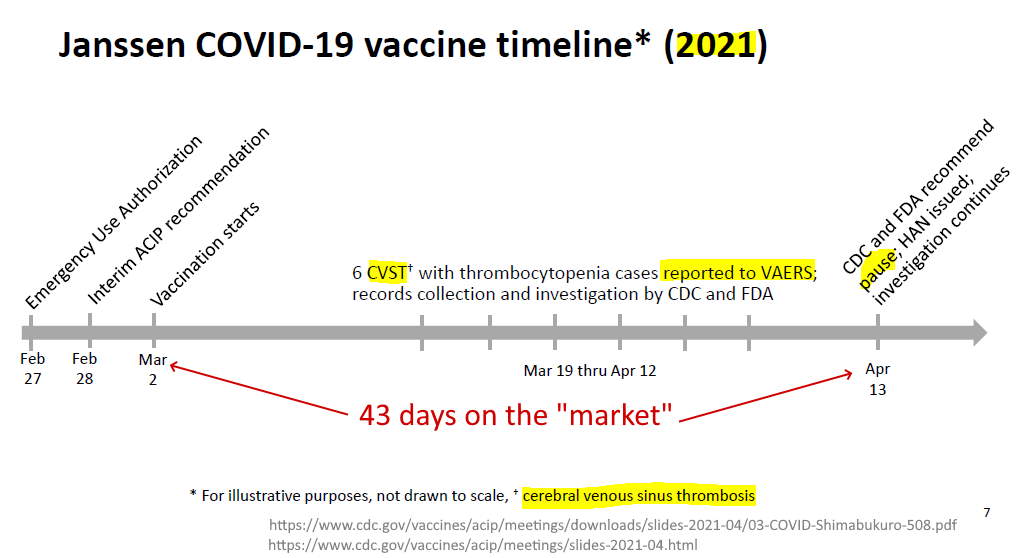
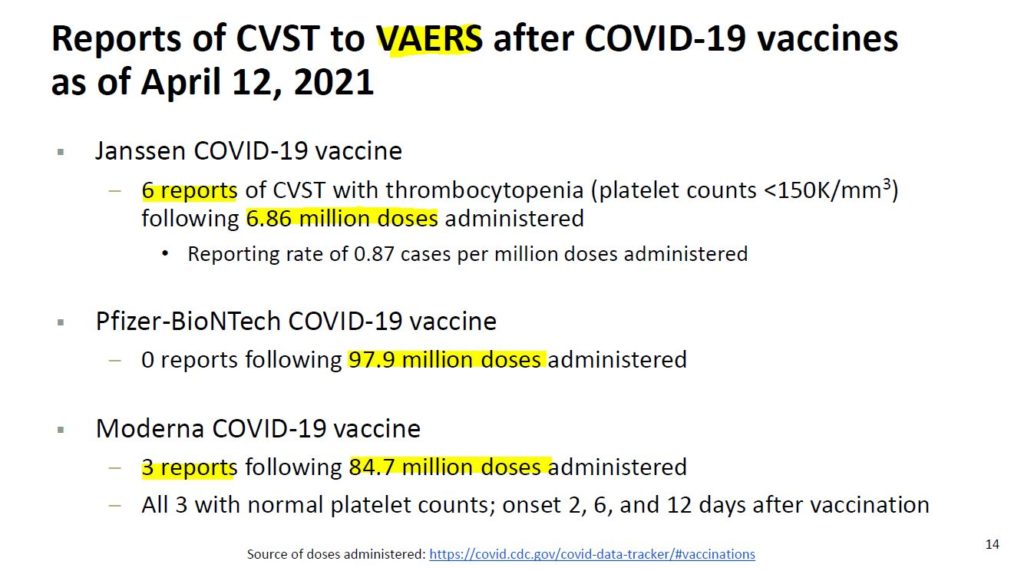
April 13, 2021 – FDA STATEMENT: Joint CDC and FDA Statement on Johnson & Johnson COVID-19 Vaccine – READ
- As of April 12, more than 6.8 million doses of the Johnson & Johnson (Janssen) vaccine have been administered in the U.S.
- Six blood clot cases to the brain were reported “In these cases, a type of blood clot called cerebral venous sinus thrombosis (CVST) was seen in combination with low levels of blood platelets (thrombocytopenia).”
- All six cases occurred among women between the ages of 18 and 48, and symptoms occurred 6 to 13 days after vaccination.
- ACIP to convene an emergency meeting the next day. “Until that process is complete, we are recommending a pause in the use of this vaccine out of an abundance of caution.”
- Federal health authorities have halted the use of the J&J COVID-19 vaccine since April 13 to investigate the rare and severe blood clots in the brain with low platelet levels that occurred in six people. – Epoch Times- READ

April 13, 2021 – CDC Health Alert: Cases of Cerebral Venous Sinus Thrombosis with Thrombocytopenia after Receipt of the Johnson & Johnson COVID-19 Vaccine – READ, ARCHIVE
April 13, 2021 – PRESS RELEASE: Johnson & Johnson Statement on COVID-19 Vaccine (Updated) – “blood clots” – READ
March 11, 2021 – PRESS RELEASE: Johnson & Johnson Announces its Single-Shot COVID-19 Vaccine Candidate Receives Positive CHMP Opinion – READ EU:”Committee for Medicinal Products for Human Use (CHMP) of the European Medicines Agency (EMA) issued a Positive Opinion to recommend the Conditional Marketing Authorization (CMA) for its single-dose COVID-19 vaccine candidate”
February 28, 2021 – PRESS RELEASE: Johnson & Johnson Announces U.S. CDC Advisory Committee Recommends First Single-Shot COVID-19 Vaccine for Adults 18 and Older in U.S. – READ
February 27, 2021 – PRESS RELEASE: Johnson & Johnson COVID-19 Vaccine Authorized by U.S. FDA For Emergency Use – First Single-Shot Vaccine in Fight Against Global Pandemic – READ, ARCHIVE
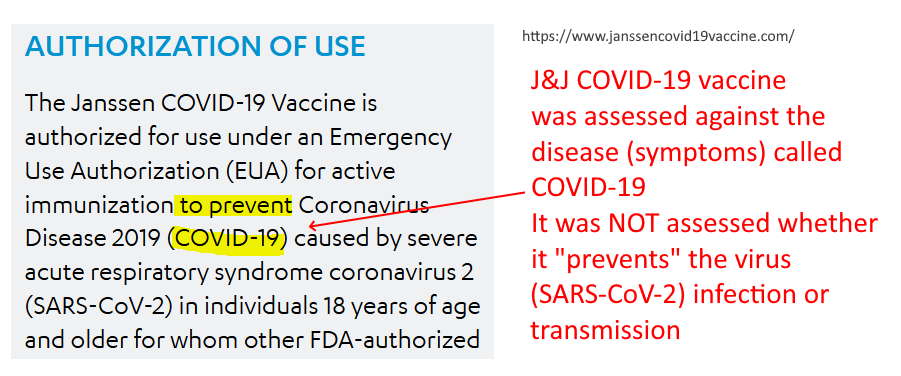
FDA Emergency Use Authorization (EUA)
- Data demonstrated protection against COVID-19 related hospitalization and death, across countries with different variants
- Available on not-for-profit basis for emergency pandemic use
- EUA follows a unanimous vote by the U.S. FDA’s Vaccines and Related Biological Products Advisory Committee (VRBPAC)
February 26, 2021 – PRESS RELEASE: Johnson & Johnson Single-Shot COVID-19 Vaccine Candidate Unanimously Recommended for Emergency Use Authorization by U.S. FDA Advisory Committee [VRBPAC] – Vote based on totality of scientific evidence provided by the Company including safety and efficacy data – READ
February 21, 2021 – Clinical Trials gov: A Study of Ad26.COV2.S in Healthy Pregnant Participants (COVID-19) (HORIZON 1) – READ, Phase II, no placebo
February 19, 2021 – PRESS RELEASE: Johnson & Johnson Announces Submission to World Health Organization for Emergency Use Listing of Investigational Single-Shot Janssen COVID-19 Vaccine Candidate – READ
February 16, 2021 – PRESS RELEASE: Johnson & Johnson Announces Submission of European Conditional Marketing Authorisation Application to the EMA for its Investigational Single-Shot Janssen COVID-19 Vaccine Candidate – READ
February 16, 2021 – Best Life: This Is Who Should Wait for the Johnson & Johnson Vaccine, Experts Say – Johnson & Johnson recently applied for emergency-use authorization from the U.S. Food and Drug Administration (FDA), which means there could be three vaccines to choose from soon – One -shot vaccine may benefit those “who have difficulty traveling to get the vaccine, particularly those with limited mobility” – READ, Live Science – READ
January 29, 2021 – Best Life: The New Johnson & Johnson Vaccine Is Only This Effective [66% effective over all], Research Shows – This new vaccine is less effective than the others, but experts say you shouldn’t discount it just yet. – ARTICLE
January 29, 2021 – PRESS RELEASE: Johnson & Johnson Announces Single-Shot Janssen COVID-19 Vaccine Candidate Met Primary Endpoints in Interim Analysis of its Phase 3 ENSEMBLE Trial (clinical trail results) – READ, ARCHIVE
- Vaccine Candidate 72% Effective in the US and 66% Effective Overall at Preventing Moderate to Severe COVID-19, 28 Days after Vaccination
- 85% Effective Overall in Preventing Severe Disease and Demonstrated Complete Protection Against COVID-19 related Hospitalization and Death as of Day 28
- based on 43,783 participants accruing 468 symptomatic cases of COVID-19, of which 34% (N= 14,672) of participants were over age 60
- “Overall serious adverse events (SAEs) reported were higher in participants who received placebo as compared to the active vaccine candidate. No anaphylaxis was observed.”
- ENSEMBLE has been funded in whole or in part by BARDA (Contract No. HHSO100201700018C) and in collaboration with NIAID
- Janssen’s single-dose vaccine candidate is estimated to remain stable for two years at -20°C (-4°F)
January 21, 2022 – CDC | ACIP committee: Use of the Janssen (Johnson & Johnson) COVID-19 Vaccine: Updated Interim Recommendations from the Advisory Committee on Immunization Practices — United States, December 2021 – READ
- Known: Cases of thrombosis with thrombocytopenia syndrome and Guillain-Barré syndrome have been reported after receipt of Janssen COVID-19 vaccine.
- On December 16, 2021, after reviewing updated vaccine effectiveness and safety data, ACIP made a preferential recommendation for the use of Pfizer & Moderna mRNA COVID-19 vaccines over the J&J for primary and booster shots in all persons aged ≥18 years in the United States.
January 14, 2021 – BestLife: These Are the Side Effects of the New Johnson & Johnson Vaccine – Authorization imminent – READ
January 13, 2021 – NEJM: Interim Results of a Phase 1–2a Trial of Ad26.COV2.S Covid-19 Vaccine – Sadoff et al – {J&J Clinical Trial data] – ARCHIVE, Updated May 31, 2021 – READ
January 12, 2021 – NY Post: Operation Warp Speed leader predicts when Johnson & Johnson will seek vaccine approval – Dr. Moncef Slaoui said that he anticipated that the pharmaceutical giant would have enough data from its late-stage trials to apply for emergency use authorization from the Food and Drug Administration in late January. – READ
2020
December 18, 2020 – PRESS RELEASE: Johnson & Johnson Announces Agreement in Principle with Gavi to Supply Janssen’s COVID-19 Vaccine Candidate to Lower-Income Countries in 2021 – up to 500 million dose – READ
December 17, 2020 – PRESS RELEASE: Johnson & Johnson Announces Its First Phase 3 COVID-19 Vaccine Trial ENSEMBLE is Fully Enrolled – READ
- Fully enrolled approximately 45,000 participants.
- Anticipate interim data by the end of January 202, “dependent on disease events”
- A separate Phase 3 clinical trial of the investigational Janssen COVID-19 vaccine candidate to explore a two-dose regimen of Janssen’s vaccine candidate (ENSEMBLE 2) is ongoing.
December 16, 2020 – CDC Advisory Committee on Immunization Practices (ACIP) made a preferential recommendation for the use of Pfizer & Moderna mRNA COVID-19 vaccines over the J&J vaccine for primary and booster shots in all persons aged ≥18 years in the United States – READ
November 4, 2020 – Clinical Trials gov: A Study of Ad26.COV2.S for the Prevention of SARS-CoV-2-mediated COVID-19 in Adults (ENSEMBLE 2) – READ
October 8, 2020 – PRESS RELEASE: Johnson & Johnson Announces European Commission Approval of Agreement to Supply 200 Million Doses of Janssen’s COVID-19 Vaccine Candidate – READ
September 23, 2020 – PRESS RELEASE: Johnson & Johnson Initiates Pivotal Global Phase 3 Clinical Trial of Janssen’s COVID-19 Vaccine Candidate – READ, The 5 Stages of COVID-19 Vaccine Development – ARTICLE
- First participants dosed in Phase 3 trial (ENSEMBLE) evaluating safety and efficacy of Janssen’s COVID-19 vaccine candidate, JNJ-78436735, also known as Ad26.COV2.S
- The initiation of the ENSEMBLE trial follows positive interim results from the Company’s Phase 1/2a clinical study, which demonstrated that the safety profile and immunogenicity after a single vaccination were supportive of further development.
- ENSEMBLE will enroll up to 60,000 volunteers across three continents and will study the safety and efficacy of a single vaccine dose versus placebo in preventing COVID-19.
- J&J continues the scaling up of its manufacturing capacity
September 3, 2020 – Johnson & Johnson Announces that Janssen’s COVID-19 Investigational Vaccine Candidate Prevents Severe Clinical Disease in Pre-clinical Studies – ARCHIVE, Nature Medicine (preprint Aug 6, 2020) – STUDY
- 20 Syrian golden hamsters vaccinated then challenged with SARS-CoV-2,
- The company’s investigational adenovirus serotype 26 (Ad26) vector-based vaccine elicited an immune response as demonstrated by “neutralizing antibodies” and prevented severe clinical disease – including weight loss, pneumonia and mortality.
- “These vaccinated animals lost less weight and had less virus in their lungs and other organs than unvaccinated control animals”…”Mortalities were absent in vaccinated animals” [all necropsied day 14!]
- “This pre-clinical study further validates our confidence in our SARS-CoV-2 vaccine candidate,”
- Phase III planned to start this month.
September 2, 2020 – Clinical Trials gov: A Study to Evaluate a Range of Dose Levels and Vaccination Intervals of Ad26.COV2.S in Healthy Adults and Adolescents [12 yrs+]- Phase II – READ
August 14, 2020 – PRESS RELEASE: Johnson & Johnson Announces Collaboration in Principle with the United Kingdom on Additional Phase 3 Study and Agreement to Supply its COVID-19 Vaccine Candidate – READ
- Agreement “in principle to collaborate with the United Kingdom of Great Britain and Northern Ireland (the UK Government) on a global Phase 3 clinical trial to explore the two-dose regimen of Janssen’s SARS-CoV-2 vaccine candidate, Ad26.COV2.S.”
August 11, 2020 – Clinical Trials gov: A Study of Ad26.COV2.S in Adults (COVID-19) – Phase I , 20-55 yrs, or healthy only 65+, 250 participants, RCT – READ, ARCHIVE
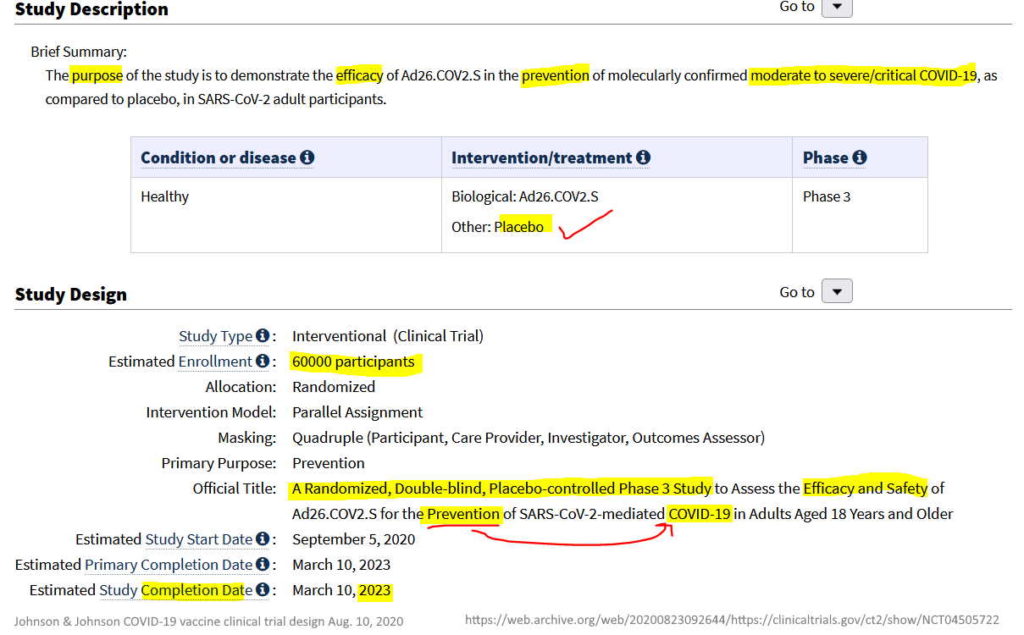
August 10, 2020 – Clinical Trials gov: A Study of Ad26.COV2.S for the Prevention of SARS-CoV-2-Mediated COVID-19 in Adult Participants (ENSEMBLE) – Sponsor: Janssen – READ, ARCHIVE, All trial changes – ARCHIVE, History of trial changes – HERE
- “The Phase 3 ENSEMBLE study is a multi-national, randomized, double-blind, placebo-controlled clinical trial in individuals 18 years of age and older.” – REF
- “The study was designed to evaluate the safety and efficacy of the Company’s vaccine in protecting against both moderate and severe/critical COVID-19 disease, with assessment of efficacy as of day 14 and as of day 28 as co-primary endpoints.” [Their trial was not designed to test for stopping infection or whether it stopped transmission]
- The study enrolled a total of 43,783 participants.
- ENSEMBLE has been funded in whole or in part by BARDA (Contract No. HHSO100201700018C) and in collaboration with NIAID – REF
August 6, 2020 – Preprint (published Nature Medicine Sep 3, 2020): Ad26 vaccine protects against SARS-CoV-2 severe clinical disease in hamsters by Tostanoski et al – READ
- “SARS-CoV-2 can infect nonhuman primates, hamsters, ferrets, hACE2 transgenic mice and other species, but clinical disease in these models has generally been mild“
- Syrian golden hamsters challenged with SARS-CoV-2,
- 20 lab animals all necropsied by day 14.
- “These data demonstrate that high-dose SARS-CoV-2 infection in hamsters led to severe weight loss and partial mortality”
- “The surviving sham controls developed potent binding and neutralizing antibody responses by day 14 after challenge”
- “SARS-CoV-2 vaccine studies in nonhuman primates have, to date, demonstrated protection against infection or reduction of viral replication in the upper and lower respiratory tracts”
August 5, 2020 – PRESS RELEASE: Johnson & Johnson Announces Agreement with U.S. Government for 100 Million Doses of Investigational COVID-19 Vaccine – READ, ARCHIVE
- Johnson & Johnson (NYSE: JNJ) (the Company) today announced its Janssen Pharmaceutical Companies have entered into an agreement with the U.S. government for the large scale domestic manufacturing and delivery in the U.S. of 100 million doses of Janssen’s SARS-CoV-2 investigational vaccine, Ad26.COV2.S, for use in the United States following approval or Emergency Use Authorization by the U.S. Food and Drug Administration (FDA)
- BARDA part of HHS’s Office of the Assistant Secretary for Preparedness and Response, in collaboration with the U.S. Department of Defense, is committing over $1 billion for this agreement.
July 30, 2020 – PRESS RELEASE: Single Dose of Johnson & Johnson COVID-19 Vaccine Candidate Demonstrates Robust Protection in Pre-clinical Studies – READ
- Study published in Nature shows J&J’s investigational SARS-CoV-2 vaccine elicits a strong immune response that protects against subsequent infection
- Based on the recently published pre-clinical data (animal studies), the Phase 1/2a first-in-human clinical trial of the J&J vaccine candidate, Ad26.COV2.S, is underway in healthy volunteers in the United States and Belgium.
July 30, 2020 – Nature: Single-shot Ad26 vaccine protects against SARS-CoV-2 in rhesus macaques – Mercado et al – READ
July 16, 2020 – Fierce Pharma: Johnson & Johnson to start human testing of COVID-19 vaccine next week – READ
- “Johnson & Johnson is revving up its plans for late-stage trials earlier than originally expected—and it’s aiming far above the FDA’s 50% efficacy bar for approval …chief scientific officer Paul Stoffels said the company has seen “strong” preclinical data, and it’s now ready to kick off human testing next week. executives said.”
July 7, 2020 – Reuters: Catalent plant in Italy will help produce J&J’s potential COVID-19 vaccine: minister – READ
July 6, 2020 – Fierce Pharma: J&J expands COVID-19 vaccine pact with Catalent for finishing work at Italian facility – READ
June 18, 2020 – Clinical Trials gov: A Study of Ad26.COV2.S in Adults (COVID-19) – Phase I/II – READ, ARCHIVE
- Study “to assess the safety, reactogenicity, and immunogenicity of Ad26.COV2.S at 2 dose levels” plus a subgroup to get a booster!
- Est 1040 participants, The placebo is not stated as saline!
- Estimated trial completion April 1, 2024
June 10, 2020 – Press Release: Johnson & Johnson Announces Acceleration of its COVID-19 Vaccine Candidate; Phase 1/2a Clinical Trial to Begin in Second Half of July – READ
- Paul Stoffels, M.D., Vice Chairman of the Executive Committee and Chief Scientific Officer, Johnson & Johnson, said, “Based on the strength of the preclinical data we have seen so far and interactions with the regulatory authorities, we have been able to further accelerate the clinical development of our investigational SARS-CoV-2 vaccine, Ad26.COV2-S, recombinant. Simultaneously, we are continuing our efforts to build important global partnerships and invest in our vaccine production technology and manufacturing capabilities. Our goal is to ensure we can deliver a vaccine to the world and protect people everywhere from this pandemic.”
- “The randomized, double-blind, placebo-controlled Phase 1/2a study will evaluate the safety, reactogenicity (response to vaccination), and immunogenicity (immune response) of the investigational SARS-CoV-2 vaccine, Ad26.COV2-S, recombinant in 1045 healthy adults aged 18 to 55 years, as well as adults aged 65 years and older. The study will take place in the U.S. and Belgium.”
June 10, 2020 – The Hill: Final testing stage for potential coronavirus vaccine set to begin in July – “the last trials for one developed by Johnson & Johnson will start in September, NIAID confirmed to The Hill.” – READ
June 10, 2020 – Fierce Pharma: Moderna, AstraZeneca and J&J coronavirus shots rev up for NIH tests beginning in July: WSJ – READ
- Johnson & Johnson has been on a manufacturing deal spree as it nears human trials for its COVID-19 vaccine hopeful…J&J has expanded its COVID-19 vaccine manufacturing pact with New Jersey-based Catalent to include work at the CDMO’s Anagni, Italy, facility.
June 5, 2020 – Fierce Pharma: After Operation Warp Speed picks 5 finalists, experts question why some vaccines were left out – READ – New York Times: Trump Administration Selects Five Coronavirus Vaccine Candidates as Finalists – READ
May 19, 2020 – New York Times: Johnson & Johnson to End Talc-Based Baby Powder Sales in North America – after facing nearly 20,000 lawsuits filed by cancer patients who claim that it’s talc was contaminated with asbestos, a known carcinogen, and that the company knew of the risks. – READ, OTHER, The Highwire – CREDIT
J&J Press Release: “misinformation” has been given as the reason for product discontinuation!- HERE,
- J&J insist they are “safe” but they lost too many lawsuits! – READ
- By August 12, 2022 they announce they will stop sales GLOBALLY in 2023! – HERE, HERE
- FDA & health websites has J&J’s back – READ, & READ
- “A 2018 Reuters investigation found that J&J knew for decades that asbestos, a carcinogen, was present in its talc products. Internal company records, trial testimony and other evidence showed that from at least 1971 to the early 2000s, J&J’s raw talc and finished powders sometimes tested positive for small amounts of asbestos.” – REF, Internal documents – HERE
- Their subsidiary files bankruptcy so cancer victims don’t get paid – READ [This is how Big Pharma always win!
May 20, 2020 – Science: DNA vaccine protection against SARS-CoV-2 in rhesus macaques – READ (part sponsored by Janssen.
May 6, 2020 – Science: Development of an inactivated vaccine candidate for SARS-CoV-2 by Gao et al (China – Sinovac Biotech) – READ, Apr 16, 2020 – PREPRINT
- Vaccine candidate (PiCoVacc) tested in monkeys
- paper referenced by Jannsen
April 29, 2020 – Fierce Pharma: J&J inks 2nd manufacturing deal to boost capacity for COVID-19 vaccine – READ
April 29, 2020 – Business Wire: Catalent Signs Agreement with Johnson & Johnson to be U.S. Manufacturing Partner for Lead COVID-19 Vaccine Candidate – READ
April 23, 2020 – Press Release: Johnson & Johnson Announces Collaboration to Expand Manufacturing Capabilities For its COVID-19 Vaccine Candidate in Support of the Company’s Goal to Supply More Than One Billion Vaccine Doses Globally – READ
March 30, 2020 – BioSpace: J&J Identifies Lead COVID-19 Vaccine Candidate, Commits to Supply 1 Billion Vaccines Worldwide – READ
March 30, 2020 – PRESS RELEASE: Johnson & Johnson Announces a Lead Vaccine Candidate for COVID-19; Landmark New Partnership with U.S. Department of Health & Human Services [BARDA]; and Commitment to Supply One Billion Vaccines Worldwide for Emergency Pandemic Use – READ, PRN
- “Johnson & Johnson began efforts in January 2020, as soon as the novel coronavirus (COVID-19) sequence became available, to research potential vaccine candidates. Research teams at Janssen, in collaboration with Beth Israel Deaconess Medical Center, part of Harvard Medical School, constructed and tested multiple vaccine candidates using the Janssen AdVac® technology.”
March 13, 2020 – PRESS RELEASE: Johnson & Johnson Announces Collaboration with the Beth Israel Deaconess Medical Center to Accelerate COVID-19 Vaccine Development – Harvard Medical School – READ
January 29, 2020 – PRESS RELEASE: Johnson & Johnson Launches Multi-Pronged Response to Coronavirus Global Public Health Threat – the Company has initiated efforts to develop a vaccine candidate against 2019-nCoV and broadly collaborate with others to screen a library of antiviral therapies. – READ
2019
July 16, 2019 – Insider: Judge To Weigh $17 Billion Opioid Case Against Johnson & Johnson – READ, CREDIT
March 14, 2019 – CNN: Johnson & Johnson hit with $29.4 million verdict in talcum powder case – 14,000 lawsuit case, internal documents showed they J&J knew their product contained asbestos – READ, CREDIT
- In 2019 J&J committed to providing up to 700,000 courses of an investigational Ebola vaccine regimen to the Democratic Republic of the Congo and Rwanda, which was developed in collaboration with Bavarian Nordic.
January 11, 2019 – Biospace News: J&J Increases Prices As More Bills Are Filed in Congress to Lower Drug Costs – “multiple companies started the new year with increases on prescription medications” – READ [Note vaccines are more profitable than drugs!]
2018
October 10, 2018 – HHS: HHS expands corporate partnership to protect against health security threats – ARCHIVE
- BARDA in partnership with Johnson & Johnson to provide $28 million over two years and up to $200 million over six years
- “first project funded will be an early development compound that can be used for acute radiation syndrome and certain potential chemical threats“
- The partnership builds on a 2017 portfolio agreement with Janssen Research & Development LLC, a subsidiary of Johnson & Johnson focused on pandemic influenza antiviral drugs and vaccines.
2017
September 26, 2017 – Regulatory Focus | RAPS Publication: Apple, Verily and J&J; Among 9 Selected for FDA Digital Health Pilot – READ
- FDA Commissioner Scott Gottlieb at AdvaMed’s MedTech conference in San Jose, California – unveiled the nine companies selected among 103 applicants to participate in the agency’s precertification [PreCert] pilot for digital health applications
- FDA launched the pre-cert pilot in July [2017] as part of the agency’s Digital Health Innovation Action Plan – PDF
- FDA Goes All Out With Digital Health Regulatory Paradigm Shift – 2018 Digital Health Pre-Cert Program [AI/Machine Lerning] – CREDIT [This tracks back to 2017 FDA Digital Health Innovation Action Plan – PDF, which began 2014 – HERE, or earlier Health Care Innovation Day 2010-2014 – ARCHIVE]
September 15, 2017 – HHS | Public Health Emergency: HHS, Janssen Research & Development join forces on innovative influenza products – A public-private partnership … – ARCHIVE
- $43 million in the first year and potentially up to $273 million over five years.
- The portfolio also will include development of an innovative influenza vaccine with the potential to protect against a broad range of seasonal and pandemic influenza viruses. The vaccine is being designed to act on a part of the influenza virus that seldom changes. If this approach is successful, the vaccine potentially could become a so-called universal vaccine.
2013
In 2013 J&J had “more than 275 operating companies in more than 60 countries employing nearly 128,000 people.” with worldwide headquarters is in New Brunswick, New Jersey, USA.