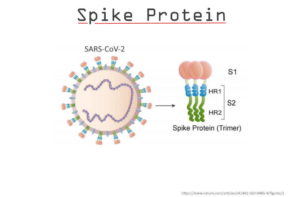
Hacked EMA data reveals potential significant CV19 mRNA batch integrity issues
On December 9, 2020 the European Medical Agency (EMA) was the subject of a cyberattack which was revealed that "COVID-19 medicines and vaccines" [1] data was stolen. This included "email screenshots, EMA peer review comments, Word documents, PDFs, and PowerPoint presentations”, of which some of those documents related to "the regulatory submission for Pfizer and BioNTech’s COVID-19 vaccine candidate, BNT162b2." [2] Nineteen days after the hack, on December 21, 2020, the EMA granted Conditional Marketing Authorization (CMA) to Pfizer-BioNTech, for the very vaccine in question - which the hack reveals that the EMA regulators had at the time over 100 regulatory objections. On of the biggest objection was reported by a BMJ investigation published on March 10, 2021, that revealed by November 23, 2020 the EMA "regulators had major concerns over unexpectedly low quantities of intact mRNA" ..."between the clinical batches and proposed commercial batches—from around 78% to 55%." Revealing concerning batch integrity instability. [3, 4, 5, 6]