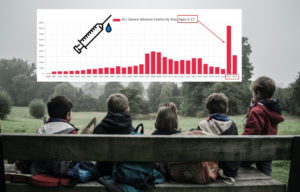
The first COVID-19 vaccine administered in America was to Sandra Lindsey, an ICU nurse, in New York City on December 14, 2020. After months of vaccine development, two companies, Pfizer-BioNTech and Moderna applied to the FDA for Emergency Use Authorization (EUA). To receive approval, the companies’ data had to be reviewed by the Centers for Disease Control and Prevention (CDC) and its Advisory Committee on Immunization Practices (ACIP), which determined that health care workers and long-term care residents should be the first groups to receive the vaccine. [1, 2, 3, 4] In a press release on December 10, 2020 the FDA assured the public that they would proceed "without sacrificing our rigorous scientific standards for safety and effectiveness." "The FDA recognizes that transparency and dialogue are critical to building public confidence in COVID-19 vaccines." "The FDA is considered the "gold standard" regulator of medical products. The process that the FDA uses to review is respected worldwide..." On December 10, 2020 The FDA's Center for Biologics Evaluation and Research’s Vaccines and Related Biological Products Advisory Committee (VRBPAC), "made up of independent scientific and public health experts from around the country", met in open session to discuss the request for EUA of..
> READ MORE