Dr Dave Martin brought attention to the fact that Dr Anthony Fauci‘s National Institute of Allergy and Infectious Disease (NIAID) was directly responsible for funding the Lipid Nanoparticle (LNP) technology used in Moderna‘s RNA product categorised as a “vaccine” and indirectly for Pfizer-BioNTech‘s equivalent product.
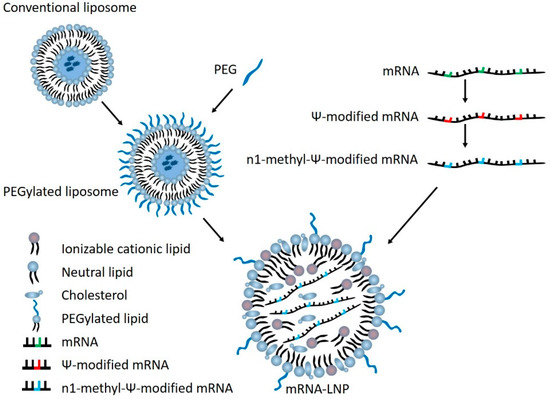
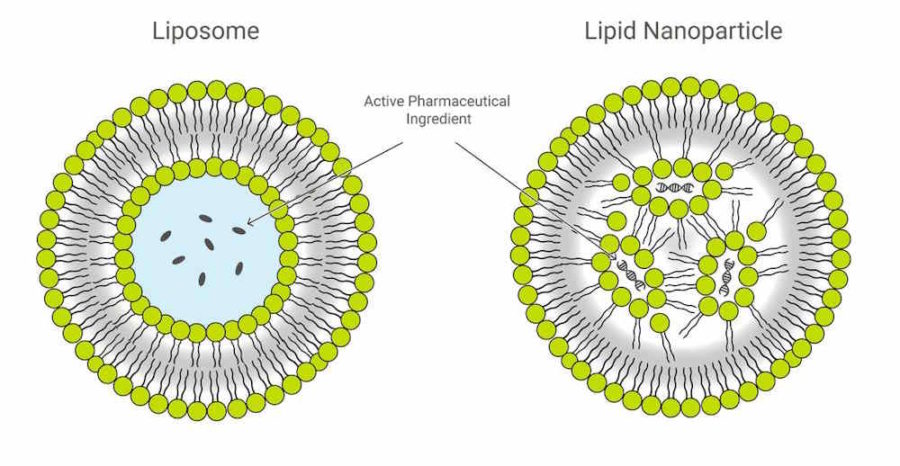
These Lipid Nanoparticle products are synthetic, man-made “fats” or lipids, which were an essential component of the gene technology “vaccines”. Their “electronic” properties encapsulate the synthetic, genetically modified, gene messenger sequences (mRNA) forming a particle on a nano scale.
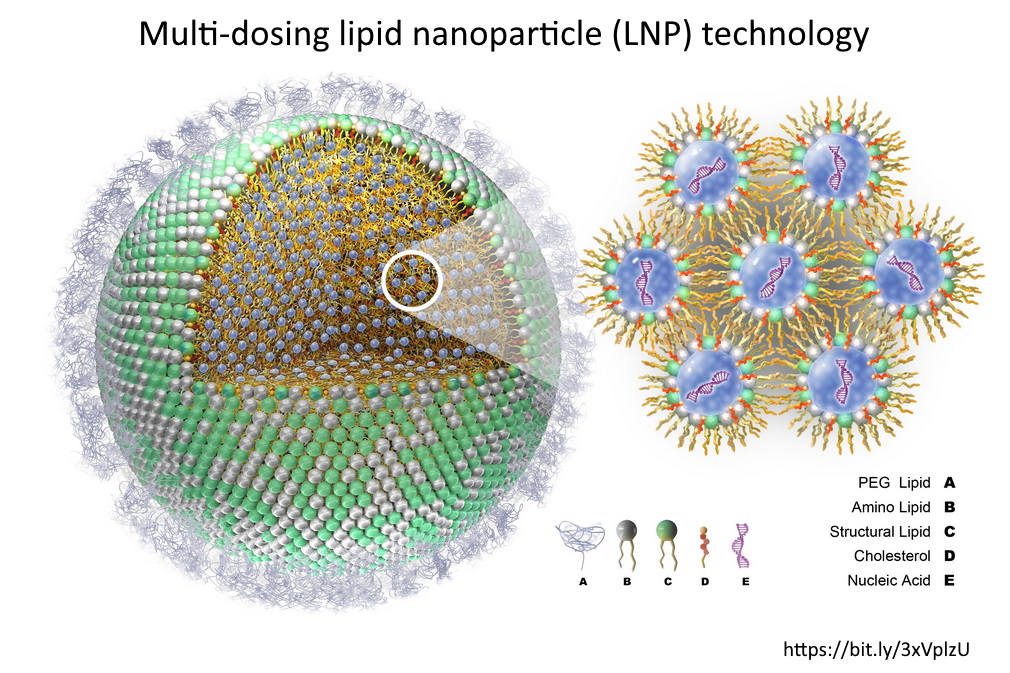
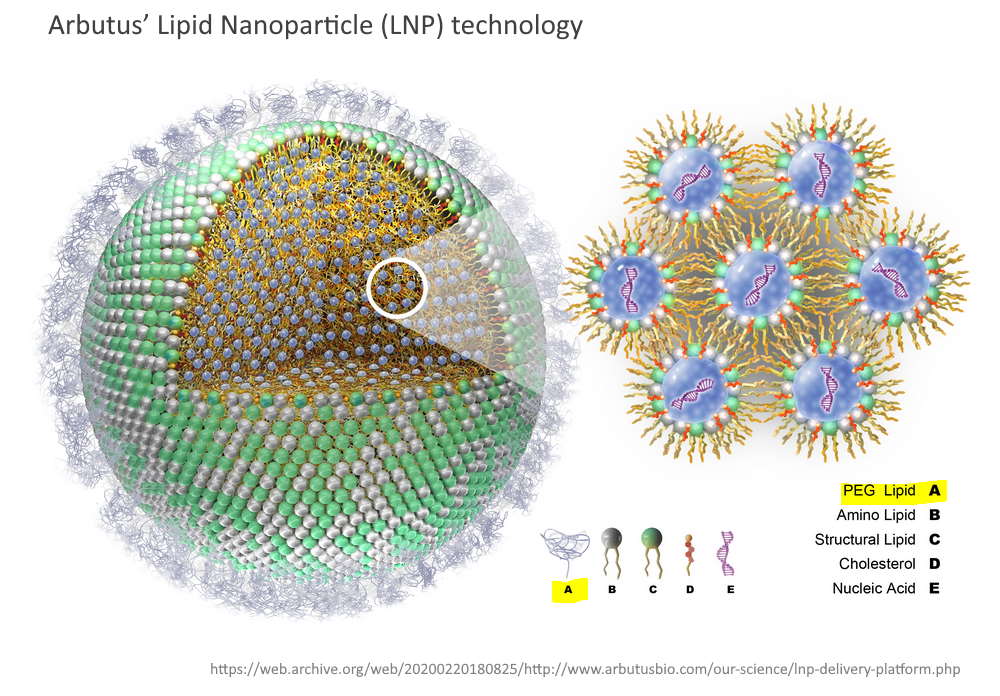
The Lipid Nanoparticle is the “Delivery Platform” for the mRNA
The role of the LNP in the “vaccine” products is two fold.
- To protect the mRNA from attack by the immune system on it’s way to the cell (pseudouridine also provides mRNA protection) and
- To facilitate entry of the mRNA gene sequence into the cell.
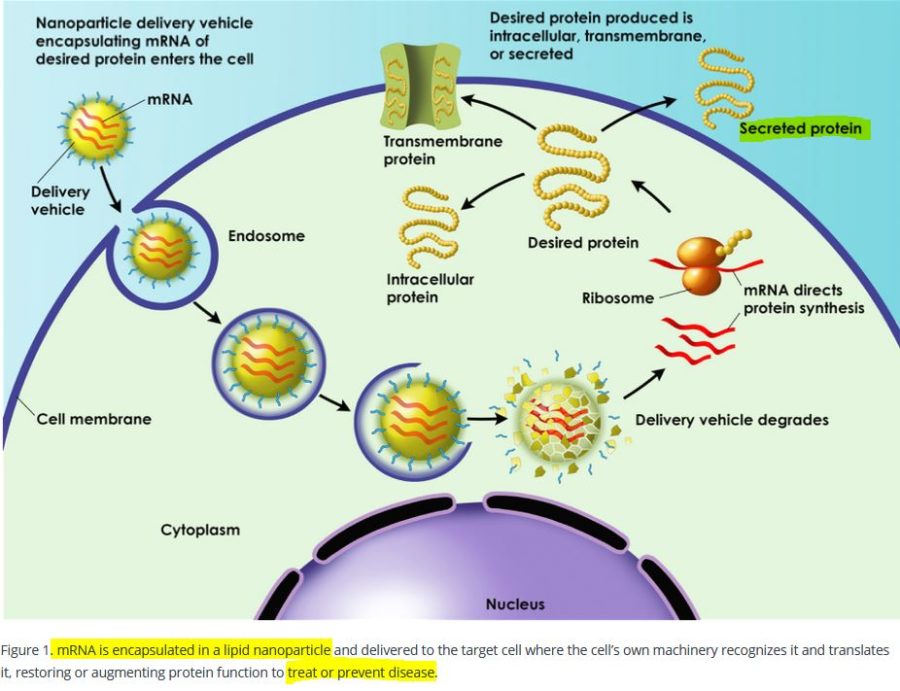
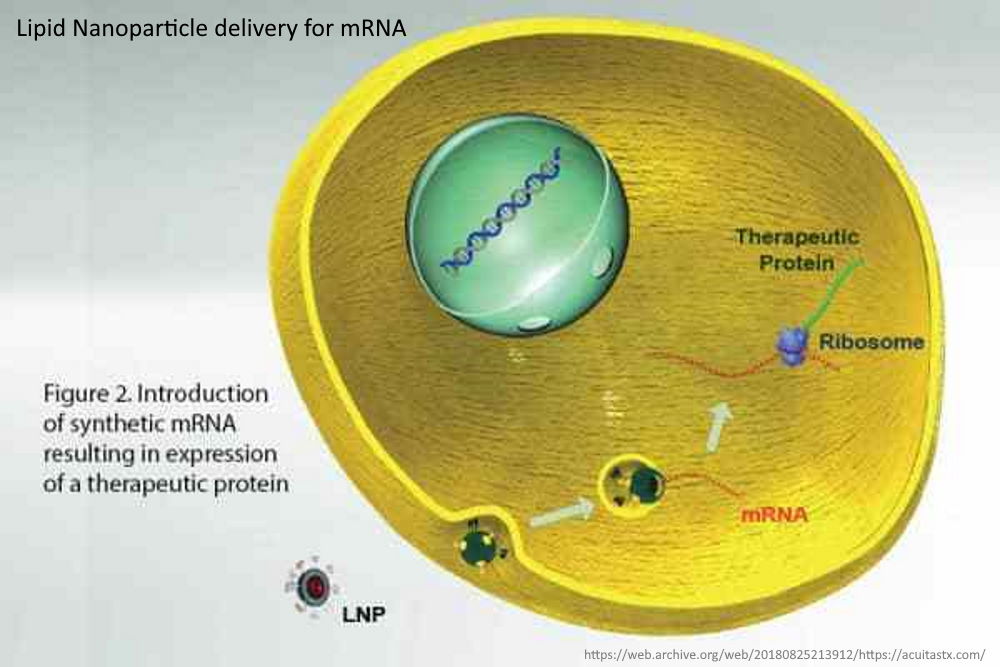
The messenger RNA has to cross the cell memberane and enter the cell cytoplasm, the LNP allows this to happen. It allows the mRNA to enter ANY cell cytoplasm – it’s not selective. Once in the cell the genetic blueprint (the mRNA) then hijacks natures cell machinery and tricks the cell into making a foreign protein – WHAT EVER protein is encoded on that mRNA “blueprint”. This is the mRNA vaccine platform and the LNP is an essential component.
It was stated in 2015 by nobel prize nominees Drew Weissman and Katalin Kariko et al, that “little is known” about the potential of using Lipid Nanoparticles (LNPs) to deliver mRNA!
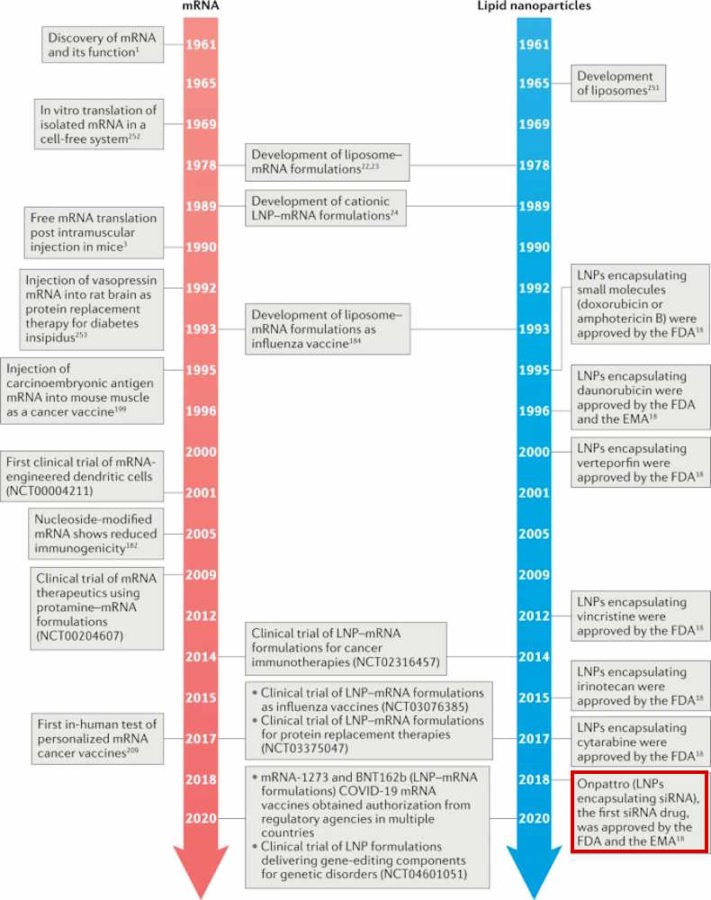
This page will attempt to capture the history and science of Lipid Nanoparticle (LNP) technology
- Pfizer-BioNTech uses Acuitus LNPs ALC-0315 and ALC-0159 in mRNA “vaccines”
- Moderna LNP as per clinical trial protocol “4 lipids (1 proprietary and 3 commercially available): the proprietary ionizable lipid SM-102; cholesterol; 1,2-distearoyl-snglycero-3 phosphocholine (DSPC); and 1 monomethoxypolyethyleneglycol-2,3-dimyristylglycerol with polyethylene glycol of average molecular weight 2000 (PEG2000-DMG).” – ARCHIVE
- 1,2-distearoyl-snglycero-3 phosphocholine (DSPC) -is used in Alnylam’s ONPATTRO™ drug formulation which gained FDA approval Aug 10, 2018. This approval grandfathered in “safety” for this molecule, but 2020 preprint stated safety data is “limited” – REF

The Fauci Dossier
In January 18, 2021 Dr Dave Martin released The Fauci Dossier [PDF], which lists the historical trail of patents including those for the Lipid Nanoparticle technology
Dr Martin provides “A sample of the convoluted flow of funds that evades public disclosure” and a prime example of a Public-Private Partnership where taxpayer money funding private companies!
U.S. Patent 8,999,351 was issued to Tekmira Pharmaceuticals Corporation in Burnaby, British Columbia. In their patent, they disclose that their research was supported by a grant from the National Institute of Allergy and Infectious Disease (Grant HHSN266200600012C). Ironically, this $23 million grant was awarded in 2006 to Alnylam Pharmaceuticals, Inc., not to Tekmira.
In 2012, Alnylam agreed to pay Tekmira $65 million to settle legal disputes including a $1 billion damages claim for “relentless and egregious” misappropriation of Tekmira’s trade secrets. From the patent filing’s earliest priority of November 10, 2008, there is no public record stating Tekmira as the beneficiary of this NIAID grant. Notwithstanding, the lipid nanoparticle technology developed from this grant is the technology now used in the Moderna COVID-19 intervention. In their 10-Q filing, Alnylam reports to have a license to technology from Arbutus – formerly Tekmira – which has accused Acuitas of misappropriating trade secrets and licensing them to Moderna and Pfizer’s collaboration with BioNTech
The Fauci Dossier by Dr David Martin (Highly referenced)
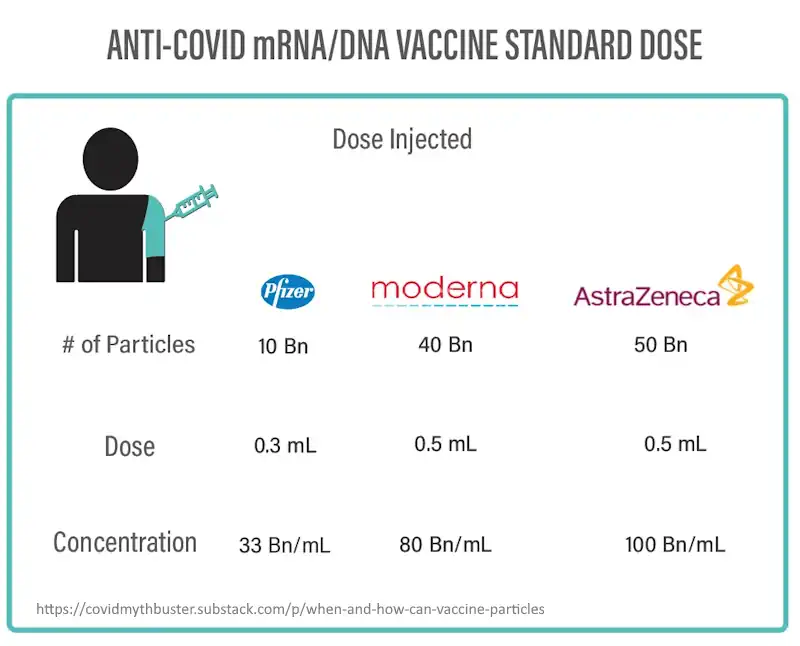
How many LNP or (viral vectors) could one injected dose deliver?: –SOURCE
LNP links in reverse chronological order
This page will continuously be updated
2024
September 10, 2024 – Senator Gerard Rennick: The TGA admits the novel cationic lipid in the Pfizer vaccine was not studied – TWEET
“Genotoxicity of the cationic lipid ALC-0315 in the Pfizer vaccine was not studied however the human dose of this nanoparticle is below the internationally agreed threshold of toxicological concern that are delivered for less than ten doses a year.”
April 18, 2024 – BioRxiv preprint: Lipid Nanoparticle-Associated Inflammation is Triggered by Sensing of Endosomal Damage: Engineering Endosomal Escape Without Side Effects – Omo-Lamai et al – READ, Jessica Rose COMMENT
February 4, 2024 (?): Bill Gates on USA Today says – Some LNP are self-assembling! – EXCERPT
Making the mRNA is really cheap and really easy and that’s the magic of this thing. But there’s no doubt in the next 5 years….we just need to mess around, there’s a lot of lipid nano particles and some are very self-assembling…
We’ll be able to build factories world wide that can make $2 vaccines with even less lead time than we’ve HAD TO HAVE HERE during this pandemic, and we’ll use those…for EVERY disease that we don’t have vaccines, we will try mRNA…so to fill in the missing vaccines.
Bill Gates
2023
September 14, 2023 – Malone Substack: Lipid Nanoparticles and mRNA Shots – Did You Take Them Without Knowing What Was in Them? – READ
- Clinical Nanotechnology is Novel and Experimental – Technically mRNA technology “formulations are associated with a wide range of particle sizes which typically vary from 100+ nanometers to a micron or more in size. ” i.e. larger than nano. “Furthermore, once formulated, the particles have a tendency to form into even larger aggregates that are often toxic but not functional for RNA delivery purposes.”…
- LNPs are Highly Complex mRNA Delivery Vehicles with a Questionable Track Record
- LNP Variability Factors and Potential Clinical Manifestations
- What about FDA Regulatory Standards for these extremely complex products that the categorised as a “vaccine”!
- Fully Taxpayer-Funded, but LNP Safety and mRNA Sequence(s) Still “Trade Secret”
- LNP Standalone Safety/Toxicity in mRNA Shots Have Never Been FDA Tested
August 17, 2023 – J of Nanobiotechnology: Emerging non-viral vectors for gene delivery – Wang et al (China) – READ
- LNP have competition! Non-viral gene delivery particles
July 28, 2023 – Modarn Life Substack: The pre-2020 BNT162 development program – Acuitas & BioNTech were waiting for Godot since at least February 2019 – READ
- BNT162 development program has been running at least since 22nd February 2019 according to FOIA documents. This is the production date of a first batch of luciferase mRNA, FK190222-01c…
- “BioNTech were making luciferase mRNAs and sending them to Acuitas to be formulated in different LNP mixtures, looking for the perfect delivery platform for systemic antigen expression creating many antibodies and thus being a good vaccine from the beginning of 2019″ – “Pilot formulations” in 2019
April 17, 2023 – Multidisciplinary Scientific Journal: The Novelty of mRNA Viral Vaccines and Potential Harms: A Scoping Review– MathewHalma, Jessica Rose & Tess Lawrie – READ, CREDIT
- “If harms are attributable to the platform itself, then regardless of the toxicity, or lack thereof, of the antigen to be expressed, the platform may be inherently unsafe, pending modification. In this work, we examine previous studies of RNA-based delivery by a lipid nanoparticle (LNP) and break down the possible aetiological elements of harm.”
- the lipid nanoparticles may have toxicity from the PEG or polysorbate 80 or from syncytia formation – REF
April 10, 2023 – ICAN PRESS RELEASE: FDA Lacks Adequate Safety Testing of Lipid Nanoparticles (LNPs) in COVID-19 Vaccines – READ
- LNPs have had very little safety testing, in part because COVID-19 vaccines are the first time LNPs have been used as an excipient in a vaccine.
- FDA hosted an event called, “NanoDay Symposium” with presenters discussing the development of drugs and vaccines containing nanoparticles, ICAN watched with interest. Unfortunately, the presentations were full of misinformation – ICAN wrote to FDA…
March 2, 2023 – Kingston Report Substack: mRNA Vaccines are a Sham. People are Being Injected with Nanotech – mRNA cationic liposome ‘vaccines’ are nanotechnologies used to introduce non-human DNA into the bodies of adults and children, forcing the directed evolution of cells inside the human body (links within) – READ
- “This tiny fat glob, known as a functional lipid, is actually one of four lipids that make up the lipid nanoparticles that go into the vaccine. Without these lipid nanoparticles, in fact, there could be no Pfizer-BioNTech mRNA vaccine. That’s because mRNA, which is the genetic material that teaches [hijacks] our cells to make the [foreign, non-human] protein that will help our immune systems produce antibodies that helps to protect us from COVID-19 [that should be SARS-CoV-2 the virus], is incredibly delicate. It needs structure and protection to do its job in the human body, and lipids provide that. The cationic lipid [electronic nanotechnology] , in particular, is the one that does the protecting. “It’s the primary component of the lipid nanoparticle, so it was needed in much larger quantities,” says [Melissa] French. If Pfizer were to manufacture the cationic lipid, itself, it would become an important component of building a reliable supply chain—and helping to protect against potential shortages—for vaccine production in the future.” – Pfizer –READ, ARCHIVE
- “BTW- a synthetic substance that carries an electronic charge is NOT a lipid. It’s a nanotechnology/electronic device.” states Kingston, [Which I agree, without the “device” or delivery aparatus, the synthetic mRNA code cannot get into the cell, which is why cationic lipid or liposome is “required”.]
- Lipofectin is the reagent of choice to integrate foreign DNA and RNA into endothelial cells. Endothelial cells are the cells that line our blood vessels, lymph nodes, and heart. – Lipofectin is allegedly – ThermoFisher Invitrogen™ product – Lipofectin™ Transfection Reagent – REF
- Lipofectin® reagent has also been shown to work well, in combination with PLUS® Reagent, for the transfection of HeLa cells

- “Nanotechnologies have been researched and developed under the guise of cancer research and for the ‘treatment’ of rare autoimmune diseases” [This is how they always do it, then the “creep” sets in! – example TGA Provisional Registration sold on treatment for cancer – TIMELINE]
January 12, 2023 – Journal of Pharmaceutical Sciences: Characterization of BNT162b2 mRNA to Evaluate Risk of Off-Target Antigen Translation by Patel et al – READ, Jessica Rose Substack critique – READ
- “Bottom line: Without the LNP [Lipid Nanoparticle delivery system], there is no mRNA technology, pseudouridines or not!”
January 17, 2023 – Journal of Pathology, Microbiology & Immunology: SARS-CoV-2 spike mRNA vaccine sequences circulate in blood up to 28 days after COVID-19 vaccination – Castruita et al – READ, Epoch Times – READ, McCullough – READ
- Abstract excerpt: “In 10 of 108 HCV [chronic hepatitis C virus] patient samples, full-length or traces of SARS-CoV-2 spike mRNA vaccine sequences were found in blood up to 28 days after COVID-19 vaccination. Detection of mRNA vaccine sequences in blood after vaccination adds important knowledge regarding this technology and should lead to further research into the design of lipid-nanoparticles and the half-life of these and mRNA vaccines in humans.”
2022
November 26, 2022 – Daily Clout: Report 46: How Many Pregnant Women Received LNP/mRNA via COVID-19 Vaccine During the Year 2021? Only Estimates Are Available – READ
October 11, 2022 – FDA NanoDay Symposium 2022 – READ, WATCH, ICAN – CREDIT
October 6, 2022 – Daily Clout: Report 44: Is mRNA-LNP Vaccine-Induced Immunity Inheritable? A Preprint Study Shows It Is – READ
September 28, 2022 – Bannons War Room: Dr. Naomi Wolf On The CCP’s Control of The Covid Vaccine, FOIA Requests Prove Vaccine Was Tested in China – WATCH, Judicial Watch shows an escalating coverup in the timeline of events – READ
- The FOIA to HHS requesting was for all biodistribution studies and data for the COVID-19 vaccines
- The newly unredacted documents reveal that the Pfizer/BioNTech COVID-19 vaccine studies, namely the vaccines’ synthetic lipid nano-particles (Acuitus: ALC-0315 [1, 2] and ALC-0159) in vitro metabolic stability studies were done in a testing facility August 2020 in Shanghai, China!
- Medicilon Preclinical Research LLC is a testing facility based in Shanghai, China! – Website: 2022-ARCHIVE, 2020-ARCHIVE
- Previously the companies name was redacted, as confidential commercial information – US govt agency hiding vaccine ties to China?
- In May 2022 the HHS knew that the LNP did not stay in the arm as the public was told. [Dr Byram Bridle warned of this in May 2021 following Japanese FOIA – find HERE]
June 28, 2022 – Biomedicines: Vaccine mRNA Can Be Detected in Blood at 15 Days Post-Vaccination – Fertig et al – READ
- Lipid nanoparticles (LNP) containing mRNA are measurable in plasma for 15 days – REF
June 6, 2022 – World Council for Health: Extremely Suspect: Bill Gates Is Funding New Injections With Lipid Nanoparticle Technology – Marik & Kory – EXCERPT
“The lipid nanoparticles were shown in preclinical studies to be profoundly pro-inflammatory, so the lipid nanoparticle itself, without a cargo, causes profound activation of the inflammatory system.”
Dr Paul Marik
May 31, 2022 – Molecular Pharmaceutics: Comparison of DLin-MC3-DMA and ALC-0315 for siRNA Delivery to Hepatocytes and Hepatic Stellate Cells – Ferraresso et al – READ
- Ionizable cationic lipids are essential for efficient in vivo delivery of RNA by lipid nanoparticles (LNPs). DLin-MC3-DMA (MC3), ALC-0315 [Pfizer], and SM-102 [Moderna] are the only ionizable cationic lipids currently clinically approved for RNA therapies.
April 22, 2022 – Dr Byram Bridle Substack | COVID Chronicles: A Moratorium on mRNA ‘Vaccines’ is Needed – Re-Visiting the Biodistribution of Lipid Nanoparticles – READ
March 4, 2022 – The Highwire | Jaxen Report: PFIZER’S COVID VACCINE DATA DUMP BEGINS – WATCH re FOIA documents which Pfizer-BioNTech submitted to FDA for BLA.
- Biodistribution study of LNP: ALC-0315 and ALC-0159 – Within 15 minutes the the LNP begin accumulating in the liver in mice, together with other organs.
2021
November 19, 2021 – Cell Journal: The mRNA-LNP platform’s lipid nanoparticle component use in preclinical vaccine studies is highly inflammatory – by Ndeupen et al – READ, Dr Ryan Cole – CREDIT
- “The LNPs could be responsible for adjuvanticity and some of the side effects”
September 1, 2021 – Molecular Therapy: Lipid-nanoparticle-encapsulated mRNA vaccines induce protective memory CD8 T cells against a lethal viral infection by Knudson, Weissman et al – READ [in mice]
August 10, 2021 – Nature Reviews: Lipid nanoparticles for mRNA delivery by Hou et al – READ
June 10, 2021 – Dark Horse Podcast: How to Save the World in Three Easy Steps – Dr. Bret Weinstein interviewed Dr. Robert Malone, the inventor of mRNA vaccine technology, and Steve Kirsch, philanthropist and tech entrepreneur (3hrs) – WATCH, BACKUP, LISTEN, CHD – ARTICLE
- Discussion re Japanese FOI biodistribution study obtained by Dr Byram Bridle which showed lipid nanoparticles from the mRNA vaccine did not stay in the deltoid muscle where they were injected as the vaccine’s developers claimed would happen, but infact circulated throughout the body and accumulated in organs and tissues, including the spleen, bone marrow, liver, adrenal glands and — in “quite high concentrations” in the ovaries.
May 28, 2021 – Trial Site News: Did Pfizer Fail to Perform Industry Standard Animal Testing Prior to Initiation of mRNA Clinical Trials? – READ
April 7, 2021 – European Chemical Societies Publishing: A Novel Graphene Quantum Dot-Based mRNA Delivery Platform by Liu et al – READ
- In recent years, graphene quantum dots (GQDs) have emerged as an attractive platform for bio-applications, involving biosensing, biomedical imaging and drug delivery.
- “Here we use graphene quantum dots (GQDs) functionalized with polyethyleneimine (PEI) as a novel mRNA delivery system.”
- “This work describes the first steps towards a potentially interesting preparation method for stable and effective mRNA delivery systems.” …”GQDs are not toxic, although cellular toxicity is a problem for these first-generation modified particles…easy to manufacture, stable and effective”
April 2021 – The biodistribution study submitted by Pfizer to the Japanese health regulatory agency: Japanese FOIA document from Pharmaceuticals and Medical Devices Agency in Japan – Pfizer study source by Dr Byram Bridle “SARS-COV-2 mRNA Vaccine (BNT162, PF-07302048) 2.6.4 – Overview of Pharmacokinetic Test” – SOURCE, ARCHIVE, Translated to ENGLISH, TWEET
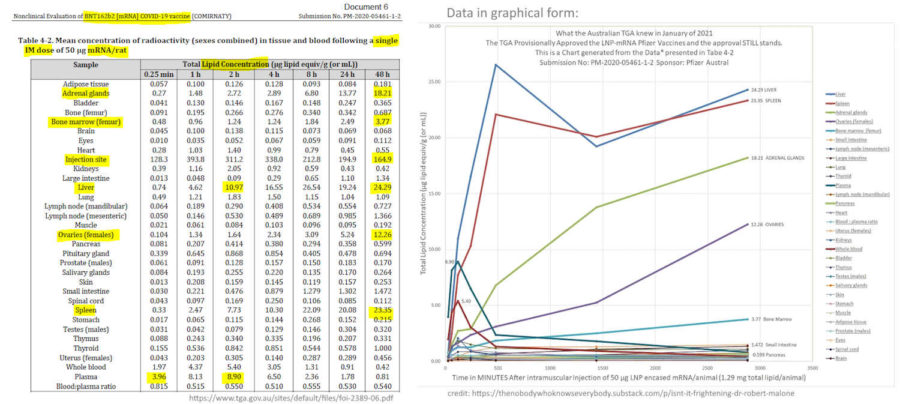
- TGA knew January 2021 – PHARMACOKINETICS document from TGA – Nonclinical Evaluation Report – BNT162b2 [mRNA] COVID-19 vaccine (COMIRNATY) – Submission No: PM-2020-05461-1-2 (obtained under Freedom of Information March 2023 – PDF, see table page 45,
- Also Arkmedic – July 2021 – TWEET, referencing this Nov 2013 paper – HERE
~August 26, 2021 – Urgent News We All Must Know by Dr. David E. Martin – WATCH
August 19, 2021 – Uncover DC: Big Pharma Cancels Scientist Behind mRNA Technology – little has been reported about how the messenger RNA technology – READ
August 18, 2021 – Forbes: Covid’s Forgotten Hero: The Untold Story Of The Scientist Whose Breakthrough Made The Vaccines Possible – Canadian biochemist named Ian MacLachlan – READ
- A months-long investigation by Forbes exposes “a complicated saga involving 15 years of legal battles and accusations of betrayal and deceit.” … “According to Forbes, the scientist most responsible for the crucial mRNA delivery method for COVID vaccines is Ian MacLachlan—a 57-year-old biochemist from Canada. MacLachlan was the chief scientific officer of two small companies, Protiva Biotherapeutics and Tekmira Pharmaceuticals.”
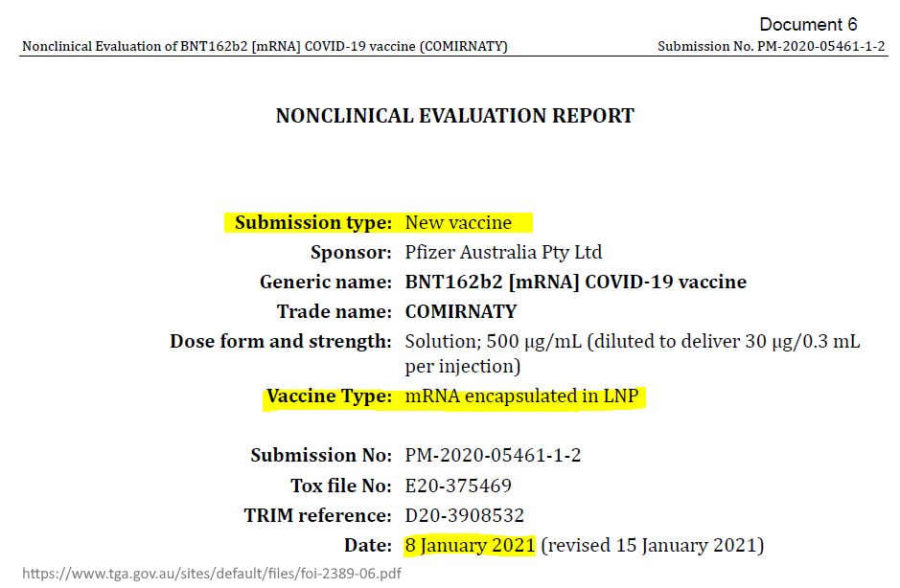
July 15, 2021 – TGA: Freedom of Information from TGA (FOI 2389) Documents relating to the evaluation of the Pfizer COVID-19 vaccine – ACCESS, ARCHIVE, CREDIT – TGA FOI documents: Pfizer BNT162b2 [mRNA] COVID-19 vaccine,
- DOCUMENT #6: Nonclinical Evaluation Report (Table 4-2), TGA knew the LNP via intramuscular injection would immediately travel throughout the body in January 8, 2021 – – PDF
- “Pfizer Australia Pty Ltd has applied for provisional registration of a new mRNA vaccine, BNT162b2 [mRNA] COVID-19 vaccine (COMIRNATY), in lipid nanoparticle (LNP) formulation [BNT162b2 (V9)] indicated for active immunisation to prevent COVID-19 disease caused by SARS-CoV-2 virus.” [what is V9?]
- “BNT162b2 (V9) was found to be immunogenic” because it “induced humoral and cellular immune responses in mice and monkeys…However, antibodies and T cells in monkeys declined quickly after 5 weeks after the second dose of BNT162b2 (V9) raising long term immunity concerns.”
- Limited pharmacokinetic studies were conducted with the LNP formulation and two novel lipid excipients (ALC-0159 and ALC-0315).
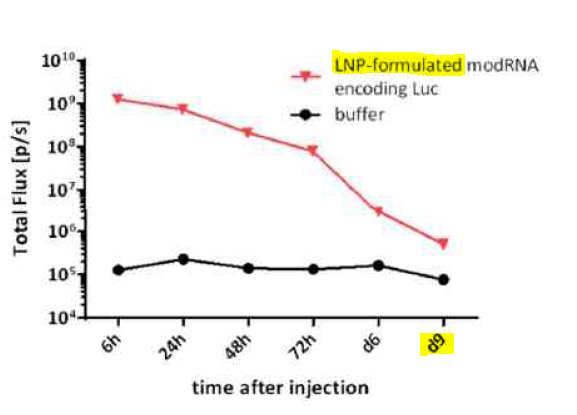
- “There are no distribution and degradation data on the S antigen-encoding mRNA” [wow!] A whole body imaging study with a surrogate, luciferase expressing mRNA indicate that the vaccine LNP formulation is expected to deliver the mRNA effectively in vivo, the mRNA and translated antigen protein are mainly localised at the injection site, distributed in liver and likely draining lymph nodes,” [not what the mouse study showed] “…and nearly completely degraded in 9 days.” [as per surrogate “luciferase signal over time” see pg 41 where Bioluminescence measurement sill reads “nearly” 106 flux at 9 days]
- “The limited studies showed slow elimination of ALC-0315 and retention in liver, and complete elimination of ALC-0159 in 14 days, with the latter eliminated in faeces most likely by biliary excretion. Both lipids are also eliminated by amide or ester hydrolysis” [what if LNP carring mRNA into the water ways, how does gene technology regulator feel about environmental contamination?, oh that’s right, they didn’t assess the products]
- “It was indicated by the sponsor that the BNT162b2 V8 and V9 variants have identical amino acid sequence with slight differences in their codon optimisation sequences for better antigen expression.”
- “The toxicity of LNP formulation or the novel excipients alone was not specifically studied…”
- “BNT162b2 COVID-19 vaccine is a nucleoside-modified mRNA based solid-lipid nanoparticle formulation to be registered for coronavirus disease. There is currently no mRNA vaccine registered in Australia.” [not correctly stated “coronavirus disease 2019 (COVID-19)“? This product was always intended as an annual jab, as stated pg17.]
- There is so much more…please read the report youreslf… (Helpful SUMMARY by Penny Butler HERE)

Vaccine Type: mRNA encapsulated in LNP
January 8, 2021 – PDF

The vaccine formulation contains two novel excipients,
2-[(polyethylene glycol)-2000] N,Nditetradecylacetamide (ALC-0159) and
((4 hydroxybutyl)azanediyl)bis(hexane-6,1-diyl)bis(2- hexyldecanoate) (ALC-0315)

June 13, 2021 – University of British Columbia: Dr. Pieter Cullis talks lipid nanoparticles and vaccines of the future on CBC Radio’s Quirks & Quarks – READ
- “One of the breakout vaccine technologies of this pandemic are the mRNA vaccines developed by Pfizer-BioNtech and Moderna. Those were some of the earliest vaccines out of the gate because of how easy they are to create ” !
- “Delivering these vaccines into our bodies would not have been possible without the work of Pieter Cullis, a professor of biochemistry and molecular biology at the University of British Columbia.”
- Q: Do you think these lipid nanoparticles will be the new standard, that they’ll replace the traditional way of delivering vaccines for viruses?
June 16, 2021 – Stew Peters Show: Dr. Carrie Madej Goes FULL TRUTH on Jab, “There’s No Off Button” – WATCH
May 28, 2021 – Trial Site News: Did Pfizer Fail to Perform industry Standard Animal Testing Prior to Initiation of mRNA Clinical Trials? – READ
May 5, 2021 – NY Times: How Pfizer makes its Covid-19 vaccine – READ
March 15, 2021 – News Medica Life Science: Research looks at inflammatory nature of lipid nanoparticle component in mRNA vaccines – READ
- “While the vital role of LNPs in these vaccines’ action is established, the potentially inflammatory nature of these LNPs is not assessed.“Because the vaccine was presumed to be non-inflammatory, these side-effects were taken to be generated from the potent immune response to the vaccine.
- Therefore, there is a need for a systemic approach to analyze the inflammatory properties of LNPs and understand their role in the vaccination process.”
March 4, 2021 – PrePrint: The mRNA-LNP platform’s lipid nanoparticle component used in preclinical vaccine studies is highly inflammatory – Ndeupen et l – READ, Published Cell Nov 19, 2021 – READ
- “…LNP inoculated mice developed rapid and visible signs of inflammation with significant elevations of inflammatory cytokines, including the signature ones, Interleukin 1 beta and Interleukin 6. In addition, thousands of genes involved in the inflammatory response were upregulated, including the CXCL series.”…”80% of those LNP inoculated mice died within 24 hours.”
- Further more “LNPs’ inflammatory properties are not site-specific; and show a fast diffusion, dispersion and distribution rate in the (other) tissues”
- “However, it will be necessary to strike a balance between positive adjuvant and negative inflammatory properties as LNP-associated vaccines move forward.”!
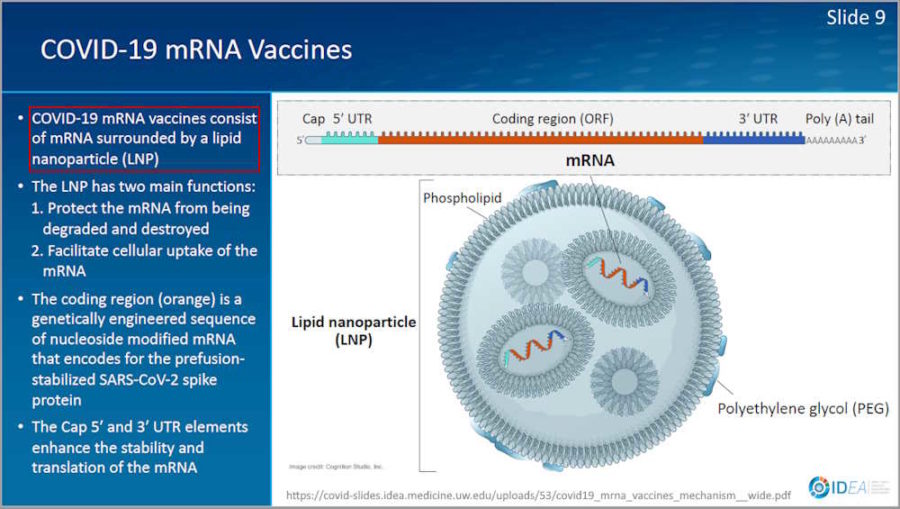
February 2, 2021- Presentation by Prof Spach Universtity of Washinton: Mechanism of Action for COVDI-19 mRNA Vaccines – Moderna and Pfizer-BioNTech – slides PDF
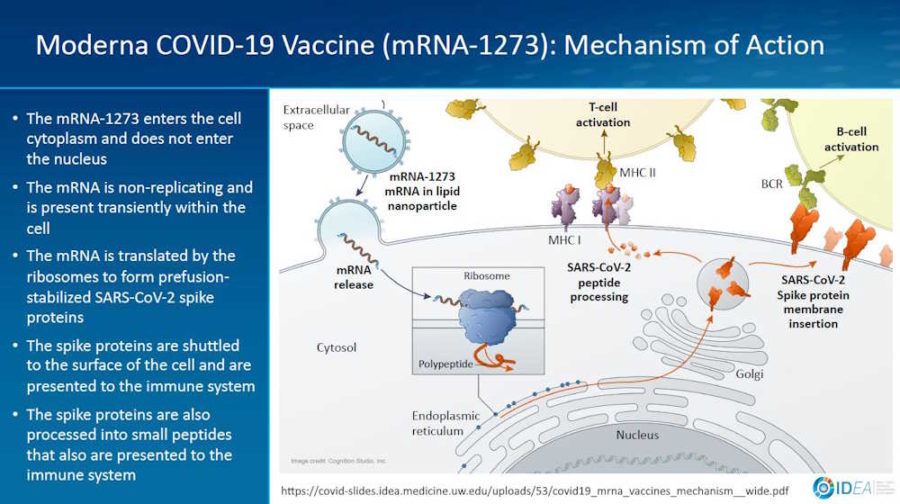
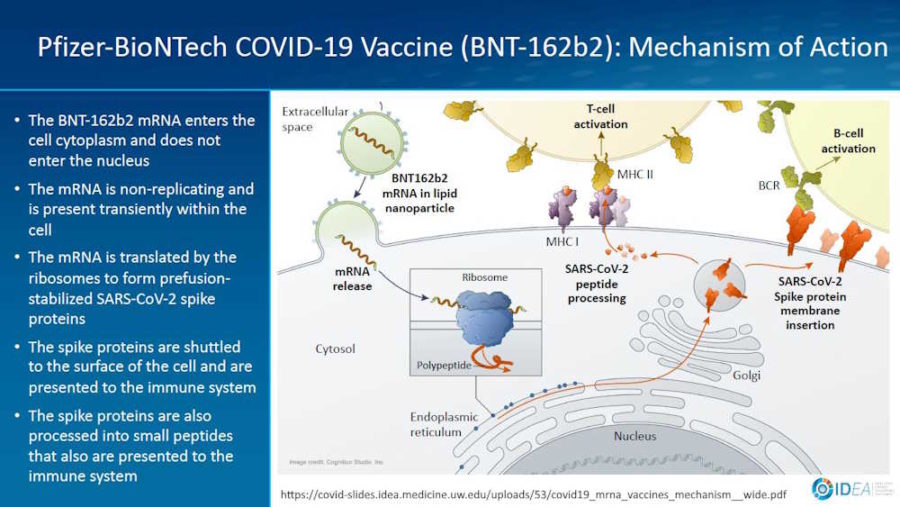
January 19, 2021 – Vaccines: Nanomaterial Delivery Systems for mRNA Vaccines – Buschmann, Weissman et al – READ
- The identity and composition of the lipid nanoparticle used in each of the vaccines was not publicly disclosed, so this paper guessed based on open source information
- Moderna LNP as per clinical trial protocol “4 lipids (1 proprietary and 3 commercially available): the proprietary ionizable lipid SM-102; cholesterol; 1,2-distearoyl-snglycero-3 phosphocholine (DSPC); and 1 monomethoxypolyethyleneglycol-2,3-dimyristylglycerol with polyethylene glycol of average molecular weight 2000 (PEG2000-DMG).” – ARCHIVE
January 18, 2021 – Butterfly of the Week: The Fauci/Covid-19 Dossier AKA The Fauci Dossier – 205 Pages, 22 Years of Research – WATCH, Dossier- PDF, YouTube, BACKUP , more on Dr Dave Martin – HERE,
2020
December 17, 2020 – MedCram w/ Prof Shane Crotty: COVID 19 Vaccine Deep Dive: Safety, Immunity, RNA Production, (Pfizer Vaccine / Moderna Vaccine) – FULL,
- Crotty equates the synthetic lipids to “butter droplets” & the pseudouridine saturated synthetic mRNA as a “temporary message”, which breaks down quickly like normal RNA !!!- EXCERPT
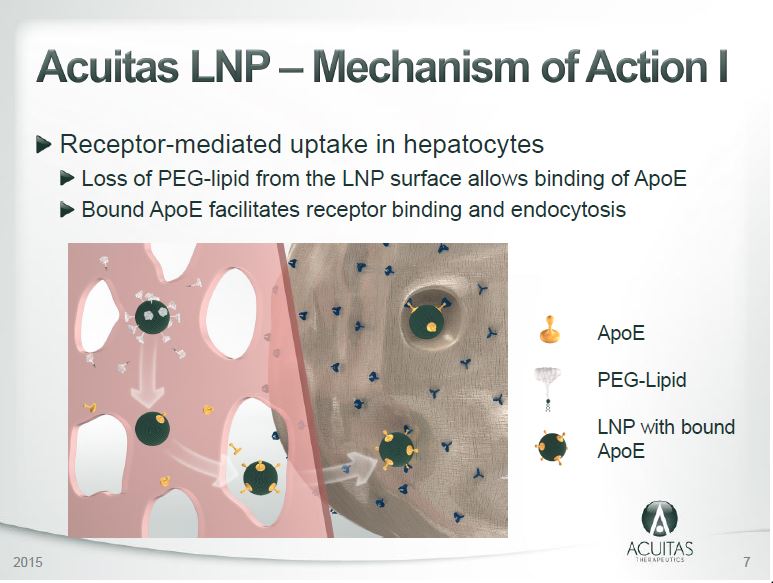
December 9, 2020 – Acuitas Therapeutics: Vancouver-based Acuitas Therapeutics Makes History with First COVID-19 Vaccine Approved for Use in Canada – PDF
- Acuitas Therapeutics has earned a global reputation for their work in developing lipid nanoparticles (LNP) – tiny “delivery vehicles” that protect the messenger RNA (mRNA) vaccine after it is injected.
- Acuitas Therapeutics (www.acuitastx.com) is Vancouver-based andwas founded in February 2009. It is a private biotechnology company that specializes in the development of delivery systems for nucleic acid therapeutics based on lipid nanoparticles.
July 27, 2020 – Evaluate: Curevac muddies the Moderna/Arbutus waters further – The markets try to figure out who really benefits from last week’s patent decision over a key mRNA technology – READ
- Arbutus admitted that it had actually signed away much of the relevant rights two years ago, and its stock lost 20%. Moderna, meanwhile, claimed that its tech was in fact “not covered by the Arbutus patents”.
- “…Curevac spelled out its reliance on a lipid nanoparticle (LNP) technology developed by Acuitas, a private Canadian company with links to Alnylam, by way of a 2016 tie-up for which it pays an annual $1.1m maintenance fee.”
- “Acuitas is at the centre of the dispute between Moderna and Arbutus, and is also the source of LNPs used by Biontech/Pfizer. The remarkable upshot of all this is that three of the major mRNA Covid-19 vaccine competitors might all in some way rely on the LNP technology in question.”
- For the sake of clarity, Arbutus might have won the legal decision, but it spells out that it has at most a 40% interest in the patent in question, US no 8,058,069” …patent now held by Genevant
- [Moderna] claims that its own LNP technology has advanced beyond what the Arbutus patents describe”
- This is because in 2018 it spun rights to the LNP technology that did not relate to hepatitis B into Genevant, a joint venture company with Riovant Sciences (owned by Vivek Ramaswamy) and Arbutus
- [Vivek is running for 2024 President!]
July 24, 2020 – BioPharma Dive: Moderna, pacing a global vaccine race, loses a key patent fight – READ
- “The U.S. Patent Trial and Appeal Board ruled against Moderna in an intellectual property dispute with Arbutus Biopharma” – who invented the mRNA delivery technology. “Moderna had argued that the technology was “unpatentable.””
- Arbutus has licensed much of the lipid nanoparticle intellectual property to a spinoff called Genevant, of which it shares ownership with Roivant Sciences.
July 23, 2020 – UNITED STATES SECURITIES AND EXCHANGE COMMISSION – FORM 8K for Arbutus Biopharma Corporation – READ
March 16, 2020 – Patent US20200385721A1 Filed: Delivering crispr therapeutics with lipid nanoparticles – Arbutus Biopharma Corp. – READ (connection with Provita Biopharmaceuticals inc )
February 20, 2020 – Arbutus BioPharma: LNP Delivery Platform – [last archive of this page?] – ARCHIVE, error page now – READ, Tekmira mirror site – READ

January 25, 2020 – Fundamental Toxicological Sciences | via Researchgate: Toxicological evaluation of DSPC (1,2-distearoyl-sn-glycero- 3-phosphocholine) – Ohgoda & Robinson – READ
2019
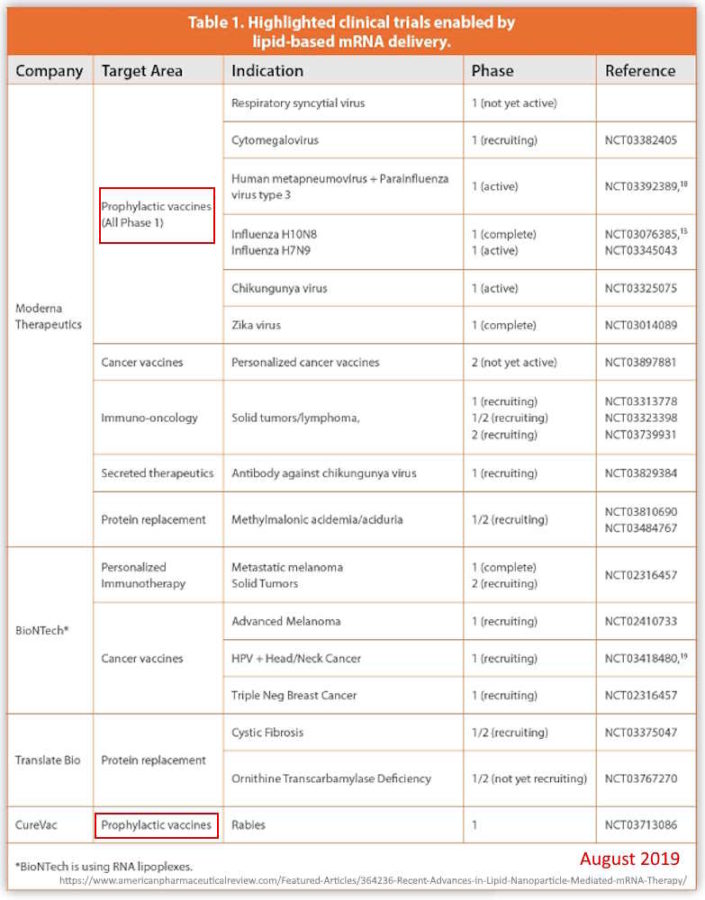
August 27, 2019 – American Pharmaceutical Review: Recent Advances in Lipid Nanoparticle-Mediated mRNA Therapy – READ [Timely article!]
- “In 2018, the FDA approved the first-ever RNA interference therapeutic product marketed as Onpattro®, a lipid nanoparticle (LNP)-based formulation of siRNA..”
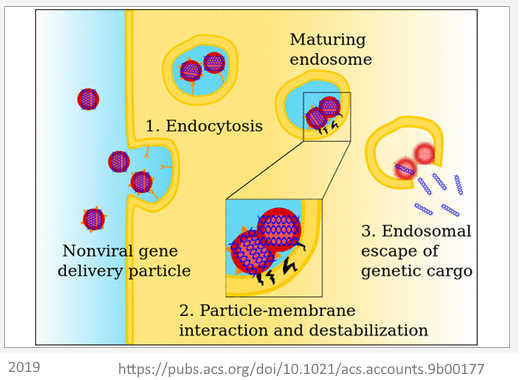
June 25, 2019 – Accounts in Chemical Research: Carriers Break Barriers in Drug Delivery: Endocytosis and Endosomal Escape of Gene Delivery Vectors by Degors et al (Netherlands, China, Pakistan) – READ, CREDIT The technology is not ready for human use yet!
- Two main classes of barriers that hinder the medical application of nanocarriers, both which may capture and destroy the “therapeutic” before it reaches their target site – the cell, These barrier are extracellular (before it gets to the cell) and intracellular (getting the LNP into the cell (endocytosis) and then triggering the release of the genetic payload into the cell cytoplasm (endosomal escape)). pH-responsive materials are discussed here. – meaning there is still more to learn June 2019!
- “PEGylation is a useful strategy to prolong the circulation time of nanoparticles in vivo by avoiding their rapid capture and clearance by undesired tissues and cells, …[thus circulate longer], it also compromises endosomal release, known as the “PEG dilemma””. The PEG layer has to be eliminated before the LNP (+ve charge) can fuse with the cell membrane (-ve charge). An acid-labile nanocarrier components may provide a potential solution (2012 paper)
- “The exact parameters that would determine and/or predict the quality of a perfect drug/gene nanocarrier, ready for clinical use, remain largely enigmatic and difficult to define. Clearly, issues of concern are stability, high-efficiency, low-cytotoxicity, large payload, potency of production scale-up, economics, etc. Even though major efforts have been undertaken over the past decades to develop nanoscale materials for the delivery of (therapeutic) molecules, for disease diagnosis and treatment, the outcome has not yet met the expectations“
June 12, 2019 – US Patent No. 10703789B2 – Modified polynucleotides for the production of secreted protein – Moderna – READ
- “A pharmaceutical composition which has a plurality of lipid nanoparticles that has a mean particle size of between 80 nm and 160 nm and contains a modified mRNA encoding a polypeptide . The lipid nanoparticles include a cationic lipid , a neutral lipid , a cholesterol , and a PEG lipid . The mRNA contains a 5 ‘ – cap , 5 ‘ – UTR , N1 – methyl – pseudouridine , a 3 ‘ – UTR , and a poly – A region with at least 100 nucleotides”
February 12, 2019 – Moderna PRESS RELEASE: Moderna Announces Positive Interim Phase 1 Data for First Combination Vaccine Against the Respiratory Viruses hMPV and PIV3 – READ, READ
- Information About Moderna’s Prophylactic Vaccines Modality: “Moderna scientists designed the Company’s prophylactic vaccines modality to prevent or control infectious diseases. …The goal of any vaccine is to safely pre-expose the immune system to a small quantity of a protein from a pathogen, called an antigen, so that the immune system is prepared to fight the pathogen if exposed in the future, and prevent infection or disease.” [Moderna’s quantity is unknown]
- This “is a combination vaccine that consists of two distinct mRNA sequences encoding the fusion (F) proteins of hMPV and PIV3 formulated in Moderna’s proprietary lipid nanoparticle (LNP) technology” – [“proprietary”???]
2018
November 16, 2018: Exelead BioPharma a contract manufacturing: Liposomes and Lipid Nanoparticles as Delivery Vehicles for Personalized Medicine – READ
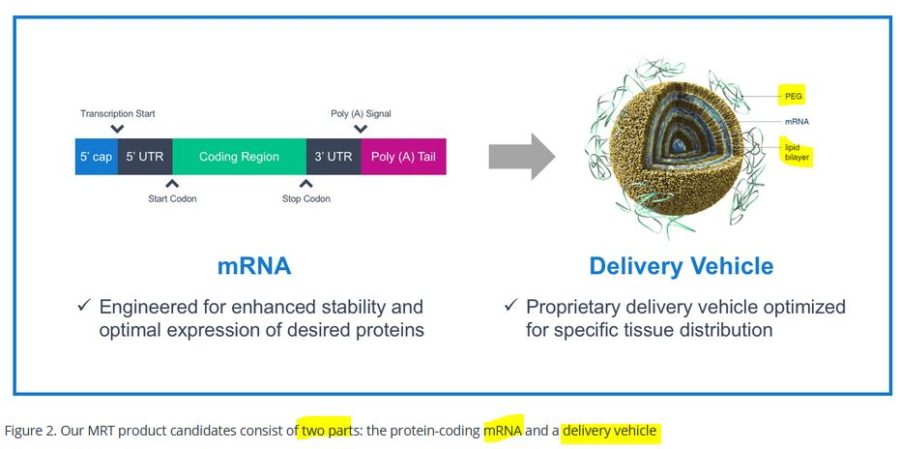
September 2018 – Translate Bio [Established 2016] – Scientific Platform: Using our proprietary mRNA therapeutic platform (MRTTM), we create mRNA that encodes functional proteins – uses LNP as carrier – ARCHIVE – They do not stipulate which LNP they use. Sanofi purchases Translate Bio in Aug 2021 – READ, though they began collaborating in 2018 – READ, [Curiously THIS article referenced it may be mistakenly as Moderna Translate Bio – I can’t find a refrence at this stage to Moderna, it maybe a typo!]
August 13, 2018 – Arbutus PRESS RELEASE: Arbutus’ LNP Licensee Alnylam Announces FDA Approval of ONPATTRO™ (patisiran), for the Treatment of ATTR Amyloidosis – ARCHIVE, PDF
- “Arbutus Biopharma Corporation (Nasdaq: ABUS), an industry-leading Hepatitis B Virus (HBV) therapeutic solutions company, today announced that the Company’s lipid nanoparticle (LNP) licensee, Alnylam Pharmaceuticals, Inc. (Nasdaq:ALNY), announced that their new drug application (NDA) for ONPATTRO, an RNAi therapeutic, has been approved by the U.S. Food and Drug Administration (FDA) for the treatment of hereditary ATTR amyloidosis with polyneuropathy.”
- “This approval also represents unprecedented clinical validation of our LNP technology which we have improved upon significantly since licensing it to Alnylam, and have recently granted broad rights to Genevant, a company we formed in the second quarter of 2018 that is jointly owned by Arbutus and Roivant Sciences. Genevant aims to advance multiple product candidates into the clinic across RNAi, mRNA, and gene editing modalities using the Arbutus LNP and ligand conjugate delivery platforms.”
- ONPATTRO Approval Triggers Royalty to Arbutus – Clinically Validated LNP Technology Now in the Hands of Genevant to Develop RNA-based Products
- “ONPATTRO is the first RNA interference therapeutic product to be approved by the FDA and represents a milestone for the technology…”
- [This is important because “unprecedented clinical valication” of Arbutus LNP used in ONPATTRO™ (patisiran) was “grandfathered” in as “proof” of safety for all future LNP registrations!]
August 10, 2018 – BioSpace: Alnylam Announces Alignment on Value-Based Agreements with Leading Health Insurers and Launches Comprehensive Patient Support Services for ONPATTRO™ (patisiran) – READ
- Approved this day by FDA – ONPATTRO™ (patisiran) is lipid complex injection, a first-of-its-kind RNA interference (RNAi) therapeutic for the treatment of the polyneuropathy of hereditary transthyretin-mediated (hATTR) amyloidosis in adults.
- Alnylam Assist™ Program Designed to Facilitate Access to ONPATTRO – [allows doctors to experience mRNA-LNP technology for the first time]
August 10, 2018 – PRESS RELEASE: Alnylam Announces First-Ever FDA Approval of an RNAi Therapeutic, ONPATTRO™ (patisiran) for the Treatment of the Polyneuropathy of Hereditary Transthyretin-Mediated Amyloidosis in Adults – READ, Bizwire – READ, FDA – READ, TIMELINE
- The FDA approval of ONPATTRO was based on positive results from the randomized, double-blind, placebo-controlled, global Phase 3 APOLLO study, the largest-ever study in hATTR amyloidosis patients with polyneuropathy. Results from the APOLLO study were published in the July 5, 2018, issue of The New England Journal of Medicine. – A “new class” of drug – FDA
- Out of 225 trial participants, 148 were given the treatment with which this BLA was based.
- Alnylam (ALN-TTR02 (patisiran)) licenced Arbutus BioPharma lipid nanoparticle technology – ARCHIVE
- Onpattro is delivered intravenously via a drip in arm – ARCHIVE
ONPATTRO™ drug formulation contains “lipid excipients (DLin-MC3-DMA, DSPC, cholesterol, and PEG2000-C-DMG)” which the FDA granted biologic License Agreement (BLA), from this moment the European regulator and likely other, do not consider these component to be “novel” any more. – Protocol REF
- DSPC and PEG are used in the LNP forumations by Pfizer-BioNTech as structural lipids, as a consequence of, but the functional lipids ALC-0315 and ALC-0159, are “novel” [1] – TIMELINE
- DSPC’s safety testing on 148 people, grandfather’s in assumed “safety” for the rest of the world
- Lipid Excipients according to abbreviations pg 27 of PROTOCOL:
- (I note the “MC3”, wondering if it is related to Acuitas’ MC3 LNP referenced slide 13 (PDF), or possibly just an abbreviation coincidence)

July 27, 2018 – PRESS RELEASE: Alnylam Receives Positive CHMP Opinion for ONPATTRO™ (patisiran) for the Treatment of Hereditary Transthyretin-Mediated Amyloidosis in Adults with Stage 1 or Stage 2 Polyneuropathy – European Commission Decision Expected in September – READ
July 23, 2018 – PRESS RELEASE: Alnylam Presents New Analyses of Clinical Results from APOLLO Phase 3 Study of Patisiran at 2018 Peripheral Nerve Society Annual Meeting – READ
July 10, 2018 – PRESS RELEASE: Arbutus Presents Corporate Update on Key Milestones – READ
- “Genevant, a recently-launched new company jointly owned by Arbutus and Roivant Sciences, announced today that it has entered into a strategic partnership with BioNTech AG, an industry leader in mRNA therapy development.”
July 5, 2018 – New England Journal of Medicine: Patisiran, an RNAi Therapeutic, for Hereditary Transthyretin Amyloidosis – Adams et al – READ, Appendix – PDF, Funded by Alnylam Pharmaceuticals; APOLLO ClinicalTrials.gov No. NCT01960348
- Based on this trial on Aug 10, 2018 the FDA approved the first use of Lipid Nanoparticle product.
April 11, 2018 – Genovant: Arbutus and Roivant Launch Genevant Sciences with Industry-Leading Platform to Develop Broad Range of RNA Therapeutics for Genetic Diseases – READ, ARCHIVE
- Genovant website propose a “Pan-RNA Approach” choose from including RNAi, mRNA, and gene editing- ARCHIVE (Genovant is jointly owned by Arbutus and Roivant Sciences
- Vivek Ramaswamy is CEO and Founder of Riovant, their formation of Genovant allows them to “deliver results through RNA solutions” – ARCHIVE
- Arbutus was formed by Roivant as a “vant” for “Working towards a cure for Hepatitis B” via vaccine – ARCHIVE , some other “vants” = Sinovant, Immunovant, Arbutus, Genovant, Cytovant, more “vants” – HERE
- Note: Arbutus Biopharma is the same as Arbutus Pharmaceuticals (www.arbutusbio.com) – REF
- “Our industry-leading lipid nanoparticle (LNP) platform has enabled clinical development of the most advanced RNA-based therapeutics. Our LNP platform is the only clinically-validated LNP delivery technology…It is the first and only LNP platform to enable an approved therapy and has enabled the first siRNA NDA submission filing through its licensed use by Alnylam Pharmaceuticals for the delivery of patisiran, a therapy to treat patients with hereditary ATTR amyloidosis.” – ARCHIVE
- Partnered with BioNTech July 10, 2018 – READ, ref ARCHIVE
- Arbutus Biopharma launched Genevant Sciences, a company jointly owned by Arbutus and Roivant Sciences (Founder & CEO Vivek Ramaswamy). – REF, REF2, Riovant news – HERE, Riovant est 2014 – ARCHIVE
- Arbutus will license exclusive rights to its LNP and ligand conjugate delivery platforms to Genevant for RNA-based applications outside of Hepatitis B virus. – REF
- “Genevant will be led by Executive Chairman Paris Panayiotopoulos, former CEO of ARIAD Pharmaceuticals through its 2017 acquisition by Takeda, who is assembling a team of RNA experts”

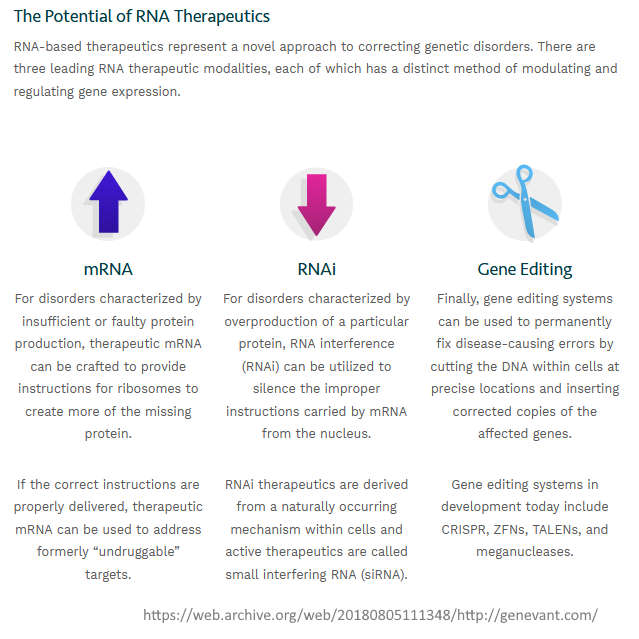
January 14, 2018 – Journal of Physical Chemistry: Structure of Lipid Nanoparticles Containing siRNA or mRNA by Dynamic Nuclear Polarization-Enhanced NMR Spectroscopy – Voger-Gravel et al – READ
- We observe that LNPs form a layered structure, and we detect that DSPC and DMPE–PEG 2000 lipids form a surface rich layer in the presence (or absence) of the cargoes and that the cholesterol and ionizable cationic lipid are embedded in the core.” [Note the DSPC and PEG are on the outside]
- “On the basis of the results, we propose a new structural model for the LNPs that features a homogeneous core with a tendency for layering of DSPC and DMPE–PEG at the surface.” [Note 8 months later the FDA approve this structure]
2017
May 4, 2017 – Acuitas Therapeutics, Inc. International Patent WO2017075531A1: Novel Lipids and Lipid Nanoparticle Formulations for Delivery of Nucleic Acids – inventor Steven Ansell – READ, PDF, CREDIT
- See page 14 for definitions of gene, gene product, lipid, cationic lipid
March 9, 2017 – Nature: Zika virus protection by a single low-dose nucleoside-modified mRNA vaccination – Pardi, Weissman et al (inc. Acuitas LNP) – READ, Acuitas – CREDIT
- “These data demonstrate that nucleoside-modified mRNA–LNP elicits rapid and durable protective immunity and therefore represents a new and promising vaccine candidate for the global fight against Zika virus”
March 2, 2017 – Nature Communications: Administration of nucleoside-modified mRNA encoding broadly neutralizing antibody protects humanized mice from HIV-1 challenge – Pardi, Kariko, Weissman et al (with Acuitas) – PDF, Acuitas – CREDIT
- “…demonstrating that nucleoside-modified mRNA represents a viable delivery platform for passive immunotherapy against HIV-1 with expansion to a variety of diseases.”
2016
December 17, 2016 – RNA Vaccines: Nucleoside Modified mRNA Vaccines for Infectious Diseases by Weissman et al – READ
- mRNA …a viable new platform for vaccine development…“Formulation of the mRNA in lipid nanoparticles (LNPs) protects it from degradation enabling high levels of protein production for extended periods of time“
April 14, 2016 – Therapeutic Delivery: mRNA vaccine delivery using lipid nanoparticles – Reichmuth et al – READmRNA vaccines are currently evaluated in clinical trials for cancer immunotherapy applications, but also have great potential as prophylactic vaccines.”
Febraury 9, 2016 – Nature: A molecular nanodevice for targeted degradation of mRNA during protein synthesis – Kyung-Ho et al – READ – Reference and diagrams to magnetic nanoparticles – for regulation of gene expression in cells?
- Why were magnets sticking to injection sites? – HERE
2015
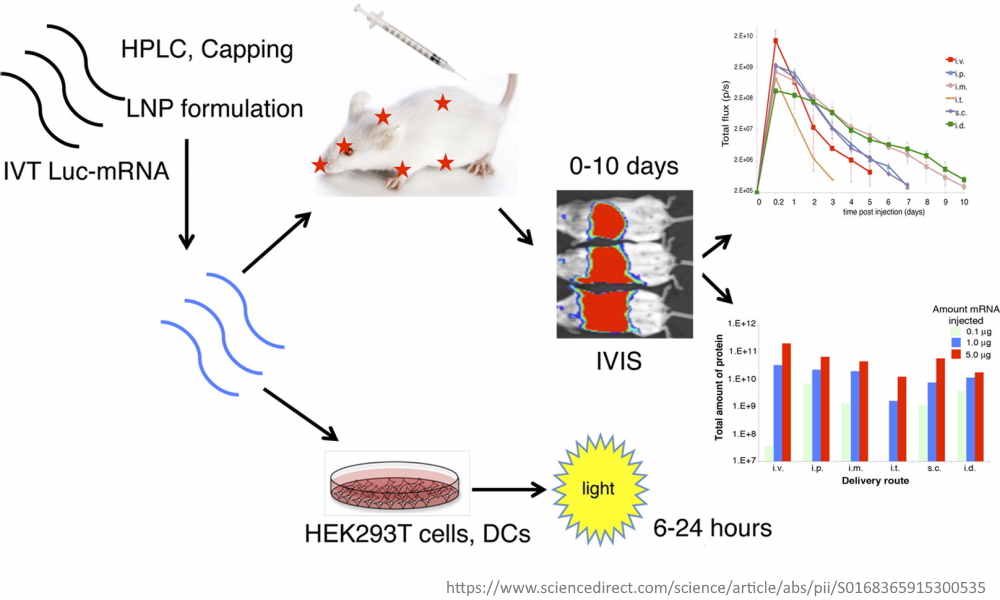
November 10, 2015 – Journal of Controlled Release: Expression kinetics of nucleoside-modified mRNA delivered in lipid nanoparticles to mice by various routes by Weissman, Kariko et al – READ [i.e.biodistribution in mice] #pseudouridine #luciferase #lipidnanoparticle
- Lipid nanoparticles (LNPs) are efficient carriers for short-interfering RNAs [siRNA] and have entered clinical trials, but “little is known about the potential of LNPs to deliver mRNA”
- “generated mRNA-LNPs by incorporating HPLC purified, 1-methylpseudouridine-containing mRNA comprising codon-optimized firefly luciferase [a luminescent enzyme] into stable LNPs”
- “mRNA translated locally at the site of injection for up to 10 days“…”active translation of the mRNA in the liver for 1–4 days” [so the mRNA does not break down quickly, and moves beyond injection site]
- “Our results demonstrate that LNPs are appropriate carriers for mRNA in vivo and have the potential to become valuable tools for delivering mRNA encoding therapeutic proteins”
October 1, 2015 – US Patent # US10415037B2 Filed: Compositions and methods for silencing hepatitis B virus gene expression – Arbutus Biopharma Corp (application granted September 17, 2019) – READ
- “The present invention also provides nucleic acid-lipid particles, and formulations thereof, wherein the lipid particles each include one or more (e.g., a cocktail) of the siRNA described herein, a cationic lipid, and a non-cationic lipid, and optionally a conjugated lipid that inhibits aggregation of particles…”
August 8, 2015 (first archived) – Arbutus Biopharma – LNP Delivery Platform – ARCHIVE, All page archives to explore – HERE
- Collaboration with US Department of Defense – ARCHIVE, Our manufacturing process is rapid, scalable, and highly reproducible ARCHIVE
August 3, 2015 – Aabutus Biopharma (NASDAQ: ABUS) Finalizes Corporate Name Change From Tekmira Pharmaceuticals – REF
- August 6, 2015 Tekmira’s CEO representing Arbutus, presents at Jefferies Hepatitis B Summit [Hep B and vaccines have a long history]
- Sept 2, 2015 – WHO: World Hepatitis Summit harnesses global momentum to eliminate viral hepatitis – co-sponsored by WHO and the World Hepatitis Alliance (WHA) – ARCHIVE WHA – WEB
July 2015 – Acuitias Non-Confidential Presentation (an update from 2013) – PDF
- Acuitas have identifid novel LNP components that provide significanly higher potentcy of mRNA expression they are MC3, ALC-0217 and ALC-0218 – (Slide 13)
- Note Acuitas Therapeutics’ lipid nanoparticles are used by BioNTech-Pfizer in their CV-19 vaccine – if the LNP run chronologically then ALC-0315 was developed later, but ALC-0159 was developed prior to 2015 [REF]
July 20, 2015 – Tekmira Announces Launch of Arbutus Biopharma, a Hepatitis B Solutions Company – REF, READ
- Tekmira Pharmaceuticals Corporation (Nasdaq:TKMR) announces plans to change its corporate name to Arbutus Biopharma Corporation (“Arbutus”, ticker symbol “ABUS”), an industry-leading therapeutic solutions company focused on developing a cure for chronic hepatitis B virus infection (HBV), to be effective on or before August 3, 2015….affirms the successful integration of OnCore BioPharma and Tekmira Pharmaceuticals into a combined company with the singular goal of delivering a cure for chronic HBV” Vancouver, Canada
- Vivek Ramaswamy is Chairman of Arbutus, he served as director of OnCore Biopharma company since August 2014, Mr. Ramaswamy is currently the President and Chief Executive Officer of Roivant Sciences, Inc., a drug development and commercialization company that is wholly owned by Roivant Sciences Ltd., a position he has held since May 2014. From August 2007 to May 2014, Mr. Ramaswamy was a member of the investment team at QVT Financial LP. – ARCHIVE, ARCHIVE
April 2015 – Arbutus Biopharm: Patisiran (ALN-TTR02) is being developed by Alnylam which “represents the most clinically advanced application of our proprietary LNP delivery technology”. – ARCHIVE
- Scientific publications date back to 2005 – LNP as a non-viral vector, through studies on Ebola, Marburg etc – ARCHIVE
2014
December 30, 2014 – Arbutus biopharma entered into a license agreement with Cytos Biotechnology Ltd – REF
~December 2014 – Riovant Sciences Inc. a wholly-owned subsidiary of Roivant Sciences Ltd. is founded by Vivek Ramaswamy a “respected healthcare investor” dating back to 2009, formally from Harvard and Yale – ARCHIVE, ARCHIVE
- Early investors page – HERE, including Dexel Pharma (est 1965) a private international specialty pharma company – ARCHIVE, that responds to crises like 2001 anthrax – ARCHIVE, and is the 2nd largest Israeli pharmaceutical manufacturer – ARCHIVE
- “Roivant Sciences, formerly Valor Biotechnology, is a biopharmaceutical firm focused on the late-stage clinical development and commercialization of nonstrategic or deprioritized drug candidates. Backed by a global multi-strategy investment firm and a global drug manufacturer, Roivant Sciences has both the capital and the in-house drug development expertise to move programs forward across a variety of pharmaceutical indications.”
November 11, 2014 – 2nd International mRNA Health Conference: POSTER: Systemic Delivery of mRNA Therapeutics using Lipid Nanoparticles (LNP): Improved Potency for Novel LNP and Influence of Route of Administration on Protein Expression by UPenn and Acuitas Therapeutics – PDF, Conference program – PDF, TIMELINE

February 2014 – Arbutus (Pharmaceutical?) enter into an exclusive, worldwide, sub-licensable license agreement with The Baruch S. Blumberg Institute (Blumberg) and Drexel University (Drexel)…- ARCHIVE
January 2014 – Journal Pharma Science: Emerging research and clinical development trends of liposome and lipid nanoparticle drug delivery systems – Kraft et al – READ“
- Lipid nanoparticles are loaded with therapeutics and may not contain an enclosed bilayer. The majority of those clinically approved have diameters of 50-300 nm. The growing interest in nanomedicine has fueled lipid-drug and lipid-protein studies, which provide a foundation for developing lipid particles that improve drug potency and reduce off-target effects.”
2013
August 2013 – Molecular Therapy: Biodegradable lipids enabling rapidly eliminated lipid nanoparticles for systemic delivery of RNAi therapeutics by Maier of Alnylam Pharmaceuticals, Cambridge, Massachusetts – READ, READ
- “The results from these early clinical trials suggest that lipid nanoparticles (LNPs), and the novel ionizable lipids that comprise them, will be important materials in this emerging field of medicine.”
- This work to “further advance the LNP platform through the development of novel, next-generation lipids that combine the excellent potency of the most advanced lipids currently available with biodegradable functionality”
August 4, 2013 – Emerging Microbes Infections: The SAM(®) vaccine platform, now in pre-clinical development, is based on a synthetic, self-amplifying mRNA, delivered by a synthetic lipid nanoparticle (LNP) by Hekele et al – READ

August 1, 2013 – Molecular Therapy: Biodegradable Lipids Enabling Rapidly Eliminated Lipid Nanoparticles for Systemic Delivery of RNAi Therapeutics – Maier et al – READ, SOURCE, context – TIMELINE
- “In recent years, RNA interference (RNAi) therapeutics, most notably with lipid nanoparticle-based delivery systems, have advanced into human clinical trials. The results from these early clinical trials suggest that lipid nanoparticles (LNPs), and the novel ionizable lipids that comprise them, will be important materials in this emerging field of medicine.”
- Biodegradability functionality of the LNP is important to assess i.e. Therapeutic Index
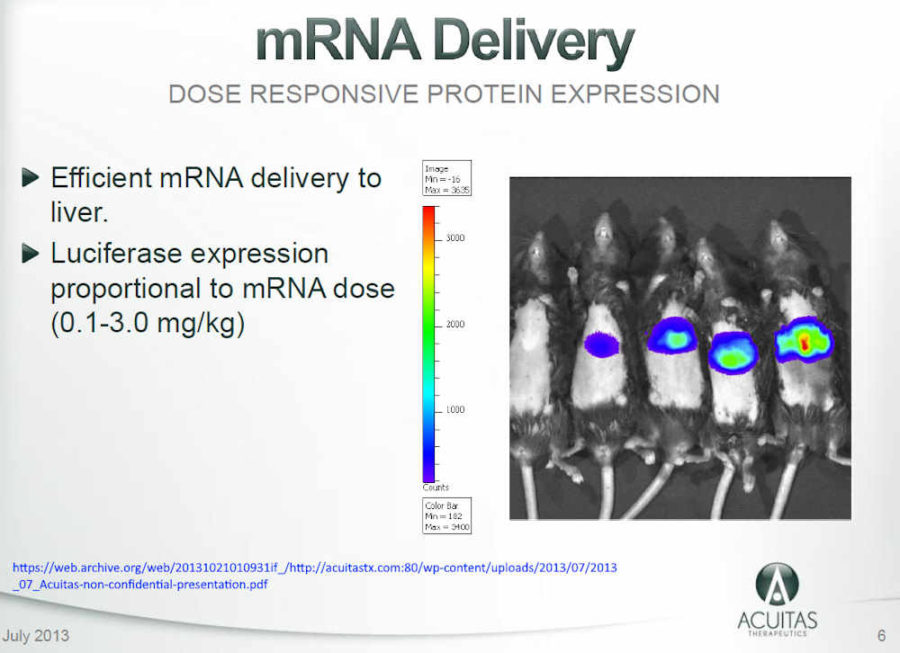
July 2013 – Acuitas Therapeutics – Non-Confidential Presentation Slides July 2013 – PDF, updated Jan 2015 – PDF, 2017 – PDF
- Acuitas Therapeutics – Lipid Nanoparticle “PROOF OF CONCEPT STUDY”: mRNA Therapeutics : Luciferase expression in liver after intravenous administration of Acuitas LNP – ARCHIVE, Presentation 2013 – HERE
- LNP Technology developed under limited license from Tekmira Pharmaceuticals Corporation – REF
- “Therapeutic messenger RNAs (mRNA) are a new class of nucleic acid drugs directed to treatment of diseases resulting from an inability to produce a key protein.
- Another application of mRNA therapeutics is in the synthesis of protein antigens for next generation vaccines.
- Therapeutic mRNAs however are very large polar molecules which are not able to enter cells by themselves. They therefore, require a delivery vehicle to access the cell cytoplasm where they can then be translated into the corresponding therapeutic protein.


March 28, 2013 – J. Control Release: Transfection efficiency and transgene expression kinetics of mRNA delivered in naked and nanoparticle format – Phua et al – READ, ARCHIVE (Acuitas claim their LNP delivery is better than this Phau et al study which was a “potent delivery system” at the time – REF
March 15, 2013 – US Patent 9539210 : Vaccine nanotechnology – Harvard, MIT, IDI et al – READ, PDF, CREDIT
“The present invention provides compositions and systems for delivery of nanocarriers to cells of the immune system…The invention provides methods of prophylaxis and/or treatment of diseases, disorders, and conditions comprising administering at least one inventive vaccine nanocarrier to a subject in need thereof..”
- January 10, 2017 – Patent assigned NIAID of National Institutes of Health, then July 22, 2019 – Patent assigned to “Immune Disease Institute Inc.” -IDI @ Harvard – ARCHIVE
- A company called SINOPEG, based in China, is the manufacturer of PEG for ALL mRNA vaccines that contain PEG, including Pfizer and Moderna – All MSDS state PEG ‘not fit for human consumption’.
- “PEG can enhance the stability and life of LNPs. SINOPEG has taken the lead in realizing the commercial production of LNP delivery materials in China.” – REF
January 17, 2013 (filed July 10, 2012) – International WO/2013/009736: COMPOSITIONS AND METHODS FOR SELF-ASSEMBLY OF POLYMERS WITH COMPLEMENTARY MACROSCOPIC AND MICROSCOPIC SCALE UNITS – READ (Referenced in Moderna patent sec. 219 “inorganic polymer” – HERE)
2012
July 10, 2012 – Journal of the German Chemical Society | Angewandte Chemie: Maximizing the Potency of siRNA Lipid Nanoparticles for Hepatic Gene Silencing In Vivo by Jayaraman et al (Funded by the Canadian Institutes for Health Research (CIHR) – Authors from AlCana Technologies (Canada) [now Acuitas] & Alnylam Pharmaceuticals (USA) & Uni BC) – READ, PDF, SOURCE, Acuitas –TIMELINE
- “Lipid nanoparticles (LNPs) containing ionizable aminolipids that self-assemble into approximately 100nm particles”
June 2012 – Biomaterials: Endosomal escape and transfection efficiency of PEGylated cationic liposome–DNA complexes prepared with an acid-labile PEG-lipid – Chan et al – READ
- Formulated acylhydrazone-based acid-labile PEG-lipid (HPEG2K-lipid, PEG MW 2000) to improve endosomal escape.
- “…PEGylated cationic liposomes containing a new acid-labile PEG-lipid were able to destabilize the endosomal membrane and induce efficient transfection” – REF
2010
December 17, 2010 – US Patent US20130017223A1: Methods and compositions for delivery of nucleic acids – University of British Columbia (UBC), Canada, Arbutus Biopharma Corp & Acuitas Therapeutics Inc – Pieter Cullis et al – READ, this patent is now abandoned
- Filed UBC, then Sept 2012 assigned to Alnylam, then 2013 Tekmira, then 2014 UBC and Acuitas, then UBC, then in 2018 to Arbutus before being abandoned!
September 2010 – Biomaterials: The systemic toxicity of positively charged lipid nanoparticles and the role of Toll-like receptor 4 in immune activation – Kedmi et al – READ
- “Non-viral gene delivery vectors are biomaterials, which are often based on cationic components. The nature, shape and charge of the biomaterials affect the interaction with the immune system.”
- “Tremendous efforts to develop carriers for nucleic acids delivery are made since the discovery of RNA interference (RNAi) in 1998″
- “…cationic nanoparticles activate toll-like receptor 4 expressed on leukocytes in a specific manner. …”
February 28, 2010 – Nat. Biotechnology: Rational design of cationic lipids for siRNA delivery by Semple et al (sponsored Tekmira Pharmaceuiticals in Canada) – READ, ARCHIVE, SOURCE, Acuitas Pharmaceuticals – TIMELINE
- Starting with “a key lipid component of stable nucleic acid lipid particles (SNALP)”, used to develop lipid nanoparticles to deliver mRNA or small RNAs
2010 – Precision Nanosystems is founded – READ, ARCHIVE , CREDIT
- Claims “simple, robust and scalable production method for LNPs encapsulating various types of nucleic acids with near 100% encapsulation efficiencies”
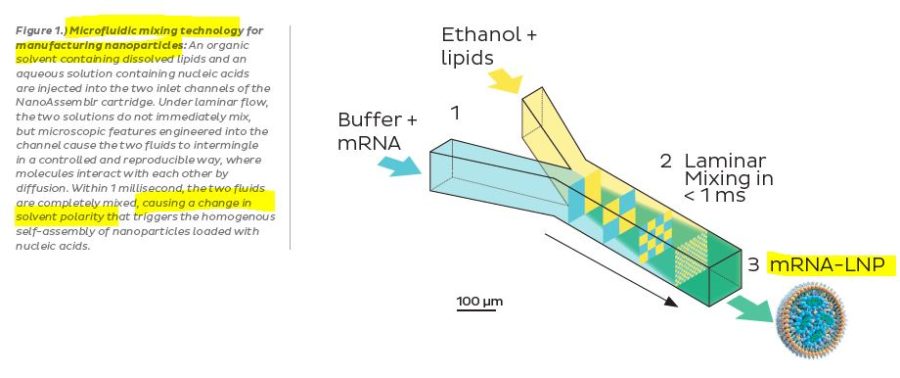
An example of a LNP manufacturing process ~2018 – PDF
2009
November 10, 2009 – US Patent No. 8999351: Lipids and compositions for the delivery of therapeutics – filed by Tekmira Pharmaceuticals Corporation (which becomes Arbutus in 2015) inventor David Butler et al – READ, [also see Sep 2006]
- “The work described herein was carried out, at least in part, using funds from the U.S. Government under grant number HHSN266200600012C awarded by the National Institute of Allergy and Infectious Diseases. The government may therefore has certain rights in the invention.” – This $23 M grant was awarded to Alnylam Pharmaceuticals, Inc. not Tekmira (same NIAID grant number) – from Fauci Dossier
- Patent US8999351B2 – 2015 Assignee = Arbutus Biopharma Corp – READ
April 15, 2009 – Patent US8058069- Application filed by Protiva Biotherapeutics Inc.: Lipid formulations for nucleic acid delivery – patent granted November 15, 2011, now Arbutus Bioparma Corp – READ, SOURCE
February 2009 – Acuitas Therapeutics (www.acuitastx.com) was founded in 2009 initially as AlCana Technologies. It is a private biotechnology company that specializes in the development of delivery systems for nucleic acid therapeutics based on lipid nanoparticles. – REF, TIMELINE
- “ACUITAS: Enabling new pharmaceutical products through nanotechnology-based research and development with Proven ability to design and synthesize key LNP components – REF, About – READ
- Applications: Protein replacement therapeutics (mRNA or plasmid delivery) and Protein knockdown therapeutics (antisense) via mRNA LNP –PDF
- Acuitas is developing LNP systems effective in vivo delivery of therapeutic mRNAs. Using mRNA encoding the reporter gene luciferase, we have successfully completed proof of concept studies and are currently developing highly potent and well-tolerated LNP carriers” – ARCHIVE, PDF
- Acuitas claim their LNPs demonstrate much greater luciferase expression in the liver after systemic administration compared to systems employed by Phua et al. (Mar 2013) –REF
- “Another application of mRNA therapeutics is in the synthesis of protein antigens for next generation vaccines. Protein antigens synthesized from mRNA inside mammalian cells (in situ synthesis) often result in better activation of the host immune system, as this resembles a normal viral infection, thereby increasing vaccine potency.” – ARCHIVE
- Acuitas R&D facilities are located on University of British Columbia campus – REF, Capabilities – READ
- “We were founded in February 2009 and have partnered with Alnylam Pharmaceuticals, the University of British Columbia, IRAP and others on several research and development programs relating to systemic delivery of nucleic acid therapeutics” – REF Formerly AlCana Technologies Inc – PDF
- Acuitas Therapeutics’ lipid nanoparticles are labelled ALC-0315 and ALC-0159 [REF] and are used by BioNTech-Pfizer in their CV-19 vaccine. These come in later years, in 2015 they working with ALC-0217 and ALC-0218 – (Slide 13) – PDF

2007
August 10, 2007 – BioProcess Online: Alnylam Awarded $38.6 Million U.S. Government Contract To Develop RNAi Therapeutics For Biological Threats – READ
- Alnylam – “awarded a $38.6 million contract over 33 months from the United States Defense Threat Reduction Agency (DTRA) to develop a broad spectrum RNAi anti-viral therapeutic for the treatment of viral hemorrhagic fever.”
July 23, 2007 – FDA: Nanotechnology Task Force Report 2007- READ, ARCHIVE, CREDIT
2006
September 29, 2006 – Technology Networks: Alnylam Awarded $23 Million U.S. Government Contract to Develop RNAi Therapeutics – READ, Fierce biotech – READ, Dr Dave Martin’s Fauci Dossier – SOURCE – Lipid Nanoparticle grant traces back to Fauci’s NIAID
- Alnylam Pharmaceuticals, Inc. announced the National Institute of Allergy and Infectious Diseases (NIAID) awarded Alnylam a $23 million federal contract (No. HHSN266200600012C) over 4-yrs to develop small interfering RNAs as anti-viral drugs targeting the Ebola virus
- With this contract, the company is establishing Alnylam Biodefense™, an initiative to build a platform for developing RNAi therapeutics targeting threats of bioterrorism.
- BUT “U.S. Patent 8,999,351 was issued to Tekmira Pharmaceuticals Corporation [see 2009] in Burnaby, British Columbia [CANADA]. In their patent, they disclose that their research was supported by a grant from the National Institute of Allergy and Infectious Disease (Grant HHSN266200600012C).” – [ref Fauci Dossier] – READ
September 27, 2006 – Fierce Biotech: Alnylam wins $23M biodefense contract – READ
- The House passed a new biodefense bill today that would create the Biomedical Advanced Research and Development Authority [BARDA] to develop therapies for pandemic viruses and biodefense attacks. The authority would “streamline” the development of new drugs – (ARCHIVE),
2005
April 2005 – Advances in Genetics Vol 53: Diffusible-PEG-Lipidi Stabilized Plasmid Lipid Particles – MacLachlan and Cullis – PDF, Arbutus publication page – SOURCE

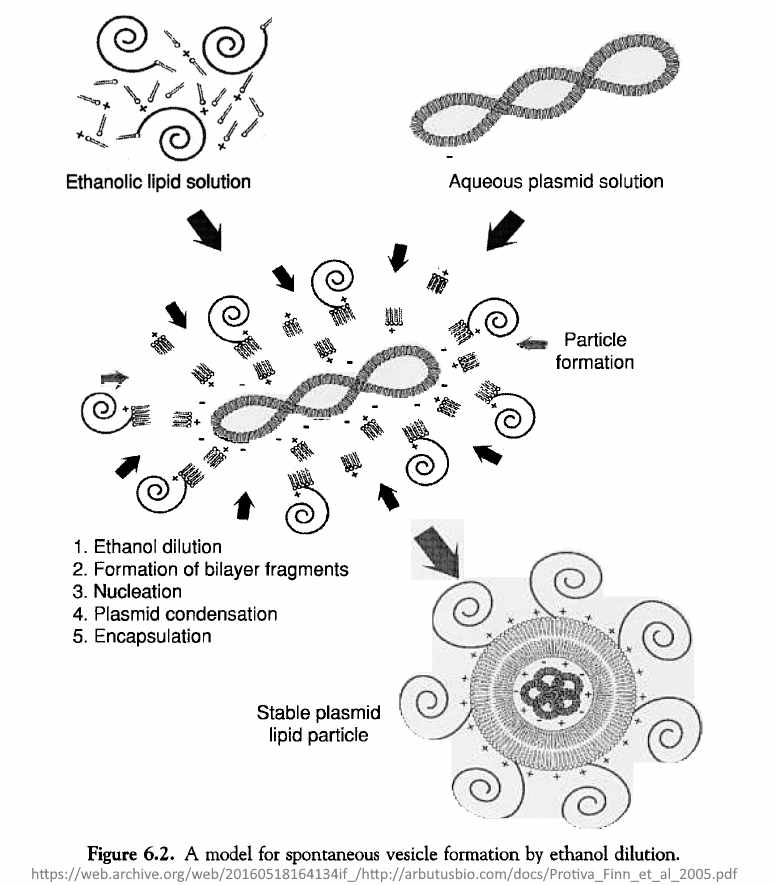
March 21, 2005 – Pharmaceutical Research: A Scalable, Extrusion-Free Method for Efficient Liposomal Encapsulation of Plasmid DNA – Jeffs, Ian MacLachlan et al – READ, CREDIT
- “Liposomes encapsulating plasmid DNA were formed instantaneously by mixing lipids dissolved in ethanol with an aqueous solution of DNA in a controlled, stepwise manner. Combining DNA-buffer and lipid-ethanol flow streams in a T-shaped mixing chamber resulted in instantaneous dilution of ethanol below the concentration required to support lipid solubility.”
- This new method will enable the scale-up and manufacture of [stabilized plasmid lipid particles (SPLP)] required for preclinical and clinical studies. Additionally, this method now allows for the acceleration of SPLP formulation development, enabling the rapid development and evaluation of novel carrier systems.
Februry 8, 2005 – US Patent : Cationic peg-lipids and methods of use by The University Of British Columbia, Pieter Cullis et al – READ, (Canada)
- …cationic-polymer-lipid conjugates (CPLs) such as distal cationic-poly(ethylene glycol)-lipid conjugates which can be incorporated into conventional and stealth liposomes or other lipid-based formulation for enhancing cellular uptake”
January 26, 2005 – FABEB Journal: Factors limiting autogene-based cytoplasmic expression systems – Finn, MacLachlan, Cullis (Canada)- READ, PDF first reference on Arbutus biopharma publications page – HERE
2003
2003 – Asklepios Biopharmaceutical Inc. (AskBio) is a private development-stage biotechnology company, which was spun out of the University of North Carolina – Chapel Hill (UNC) Biotech Centre. AskBio is “engageded in the development and delivery of novel protein and cellular based therapies through design of proprietary Biological Nano ParticlesTM (BNP)” – ARCHIVE, website ARCHIVES, Phase 1 muscular dystrophy trial 2006- CREDIT, CREDIT
- 2006 press release: “AskBio is engaged in the development of novel, intracellular protein therapeutics using its BNPTM technology platform and Self-Complementary vector technology. BNPsTM may be used to target delivery of a broad variety of biological material, including therapeutic genes, RNAi, and vaccines, among others, to specific tissue.” – ARCHIVE
- BNT platform technology was developed at UNC Chapel Hill ~2000 – REF, AskBio today, state started in 2001 – WEB
2002
April 30, 2002 – Patent Application WO2002087541A1 filed: Lipid-based formulations for gene transfer – by Provita Biotherapeutics, Inventor Ian Maclachlan – READ, CREDIT, more Mcclauchlan patents – HERE
May 12, 2002 – New Scientist: DNA nanoballs boost gene therapy – ARCHIVE, CREDIT
- “Scrunching up DNA into ultra-tiny balls could be the key to making gene therapy safer and more efficient. The technique is now being tested on people with cystic fibrosis.”
- “At around 100 nanometres in size, most liposomes are too large to pass through the tiny pores in the nuclear membrane except when the membrane breaks down during cell division. Even if cells are rapidly dividing, delivering genes via liposomes is not very efficient…But researchers at Case Western Reserve University and Copernicus Therapeutics, both in Cleveland, Ohio, have developed a way to pack DNA into particles 25 nanometres across, small enough to enter the nuclear pores.”
- These nano-scale lipids carried DNA into cell nucleus – now think DNA contamination in Pfizer’s mRNA platform products?
2000
January 21, 2000 – The Nanotechnology Revolution begins – TIMELINE
- President Clinton launched the National Nanotechnology Initiative (NNI), which will lead the “next industrial revolution”….read more on TIMELINE
1998
May 14, 1998 – US patent US6287591B1 Filed: Charged therapeutic agents encapsulated in lipid particles containing four lipid components – University of British Columbia – Steven Ansell, Pieter Cullis et al – READ, Expired 2017 –
- “A61K9/1272 – Non-conventional liposomes, e.g. PEGylated liposomes, liposomes coated with polymers with substantial amounts of non-phosphatidyl, i.e. non-acylglycerophosphate, surfactants as bilayer-forming substances, e.g. cationic lipids”
- Curiously Steven Ansell Uni BC, is also submitting patents for “System and method of looking up and validating a digital certificate in one pass” – READ
- Steven Ansell of Uni BC will become affiliated with Acuitas Therapeutics, Inc., Alnylam Pharmaceuticals Inc, Arbutus Biopharma Corp and Tekmira Pharmaceuticals
1996
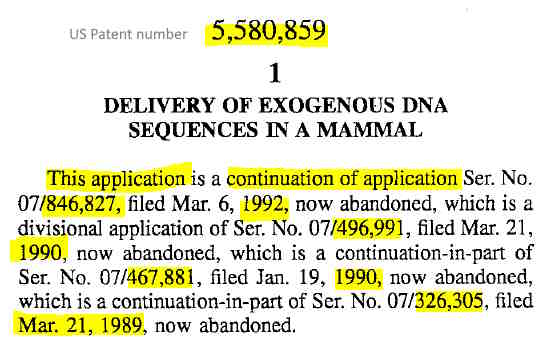
December 3, 1996 – US Patent 5580859: DELIVERY OF EXOGENOUS DNA SEQUENCES IN A MAMMAL, Robert Malone et al, VICAL Incorporated (Filed March 18, 1994) – READ, PDF cationic liposomes (later lipids) are “requied”
- In the embodiments of the invention that require use of liposomes, for example, when the polynucleotide is to be associated with a liposome, it requires a material for forming liposomes, preferably cationic or positively charged liposomes, and requires that liposomal preparations be made from these materials.
1989
May 12, 1989 – Proc. Natl. Acad. Sci. USA (PNAS):: Cationic liposome-mediated RNA transfection – Robert Malone et al – READ, PDF, CREDIT
- Dr. Robert Malone‘s invented stabilized nucleic acid by packaging it in a liposome with a positive charge. A liposome is a lipid sac that can carry drugs or other substances like mRNA into tissues.
- “We have developed an efficient and reproducible method for RNA transfection, using a synthetic cationic lipid, N-[1-(2,3-dioleyloxy)propyl]-N,N,N-trimethylammonium chloride (DOTMA), incorporated into a liposome (lipofectin)”
- Lipid nanoparticles (cationic or positively charged) are new and improved delivery devices based on Malone’s technology
March 21, 1989 (filed) – US Patent 07/326305: EXPRESSION OF EXOGENOUS POLYNUCLEOTIDE SEQUENCES IN A MAMMAL (abandoned 1991) Robert Malone et al – READ, referenced in US Patent 5580859 1996 – HERE
1986
January 6, 1986 (file date) – EUROPEAN PATENT APPLICATION 0187702: N-(omega,omega-1-dialkoxy)- and N-(omega,omega-1-dialkenoxy)-alk-1-yl-N,N,N-trisubstituted ammonium surfactants, their preparation and pharmaceutical formulations containing them. – READ, referenced in Dr Malone’s US Patent 5580859 – HERE
- This invention relates to glycerol-based cationic compounds.
- [I’m not a chemist but there are similarities in Lipofectin formula – just as a reference READ,
Lipofectin is refrenced by Karen Kingston – HERE]- “Lipofectin® reagent has also been shown to work well, in combination with PLUS® Reagent, for the transfection of HeLa cells. Lipofectin® Transfection Reagent is a 1:1 (w/w) liposome formulation of the cationic lipid N-[1-(2,3-dioleyloxy)propyl]-n,n,n-trimethylammonium chloride (DOTMA) and dioleoyl phophotidylethanolamine (DOPE) in membrane-filtered water.”
- Note HeLa are immortal cell line (tumor/cancer) used in scientific research cell lines, they are listed in the patent on the Moderna website for all of the mRNA vaccines accoring to Karen Kingston – HERE