Dr Peter Doshi, PhD, is an associate professor in the Department of Practice, Sciences, and Health Outcomes Research at the University of Maryland School of Pharmacy. He is also an associate editor at the British Medical Journal (BMJ). [1, 2]
By 2009 “Dr. Doshi, after receiving undergraduate and master’s degrees in anthropology and East Asian studies from Brown and Harvard, had shifted focus and was pursuing a doctorate at M.I.T., studying the intersection of medicine and politics.”
His research focuses on the drug approval process, how the risks and benefits of medical products are assessed and communicated, and improving the credibility and accuracy of evidence synthesis and biomedical publications. Doshi campaigns for greater transparency of clinical trial data…”
Following the COVID-19 vaccine roll out he began to publish his concerns about “The Science” surrounding these new technology injectable products. “Doshi pointed out numerous red flags that were never discussed or addressed by the medical community.”
This page captures some highlights of Dr Doshi’s work, both during the COVID-19 pandemic, as well as the time period form the previous pandemic where he questioned the “definition of pandemic” and the efficacy of the flu vaccines, and transparency of data.
Links in reverse chronological order
2024
February 2024 – J. Eval. Clin. Practice: Sources of bias in observational studies of covid-19 vaccine effectiveness – Fung, Jones and Doshi – READ
- The lack of critical discussion [of the limitations of the methodologies of these early observational studies] is notable, for even highly effective vaccinations could only partially explain the drop in rates of covid-19 cases, hospitalisations, and deaths by mid-2021.”
2022
August 31, 2022 – Elsevier: Serious adverse events of special interest following mRNA COVID-19 vaccination in randomized trials in adults – Faiman, Doshi et al – READ, Vaccine – READ
- In 2020, prior to COVID-19 vaccine rollout, the Brighton Collaboration created a priority list, endorsed by the World Health Organization, of potential adverse events relevant to COVID-19 vaccines. We adapted the Brighton Collaboration list to evaluate serious adverse events of special interest observed in mRNA COVID-19 vaccine trials.”
- “In March 2020, the Brighton Collaboration and the Coalition for Epidemic Preparedness Innovations partnership, Safety Platform for Emergency vACcines (SPEAC), created and subsequently updated a “priority list of potential adverse events of special interest relevant to COVID-19 vaccine trials.”” –
- Safety Platform for Emergency vACcines – SO2-D2.1.3 Priority List of COVID-19 Adverse events of special interest – PDF
- “Full transparency of the COVID-19 vaccine clinical trial data is needed to properly evaluate these questions. Unfortunately, as we approach 2 years after release of COVID-19 vaccines, participant level data remain inaccessible”
April 6, 2022 – FDA’s Vaccines and Related Biological Products Advisory Committee (VRBPAC) MEETING – Data Integrity and Regulatory Oversight presented by Peter Doshi – EXCERT, FULL, CREDIT
- Doshi presents information about Pfizer trial Whistleblower, Brook Jackson , and reveals with these massive unblinding concerns from Pfizer’s clinical trials that the FDA never inspected Ventavia.
- FDA only inspected 9 of 150+ Pfizer trail sites, before approving, and inspected only 1 Moderna site.

January 21, 2022 – Uncover DC: BMJ Demands Raw Data “Now” on COVID-19 Vaccines & Treatments – READ
January 19, 2022 – BMJ Editorials: Covid-19 vaccines and treatments: we must have raw data, now Doshi et al – READ,
- Data should be fully and immediately available for public scrutiny
2021
December 23, 2021 – BMJ | Analysis: Evaluating covid-19 vaccine efficacy and safety in the post-authorisation phase – Doshi et al – READ
- Covid-19 vaccines were widely administered following “conditional” authorisation based on short clinical trials, when important questions remained unanswered…etc
- “Doshi penned an editorial that noted that while a variety of additional research trials were legally required in return for receiving an EUA (Pfizer agreed to do 13 and Moderna 8), many had not been done, and basic pieces of information such as the study protocols were only available for 5 of Pfizer’s studies and 5 for Moderna. Most of the protocols that were available were only available because the European Medical Agency legally required them to be visible, something the more corrupt FDA does not require pharmaceutical companies to do.” – REF
November 1, 2021 – Sen. Johnson Holds Expert Panel On Federal Vaccine Mandates and Vaccine Injuries – FULL, Press Release – READ – Dr. Peter Doshi testimony PART 1, PART 2, BACKUP, EXCERPT, Commentary – READ
- U.S. Sen. Ron Johnson held a panel discussion in Washington, D.C., with doctors and medical researchers who treat COVID-19 vaccine injuries, along with patients who have experienced adverse events due to the COVID-19 vaccine.
- Dr. Peter Doshi gives evidence at an expert panel on COVID vaccine mandates & injuries. – TWEET
- What’s Under the Hood of Pfizer Clinical Trials Is Not Science – It’s Business” – EXCERPT
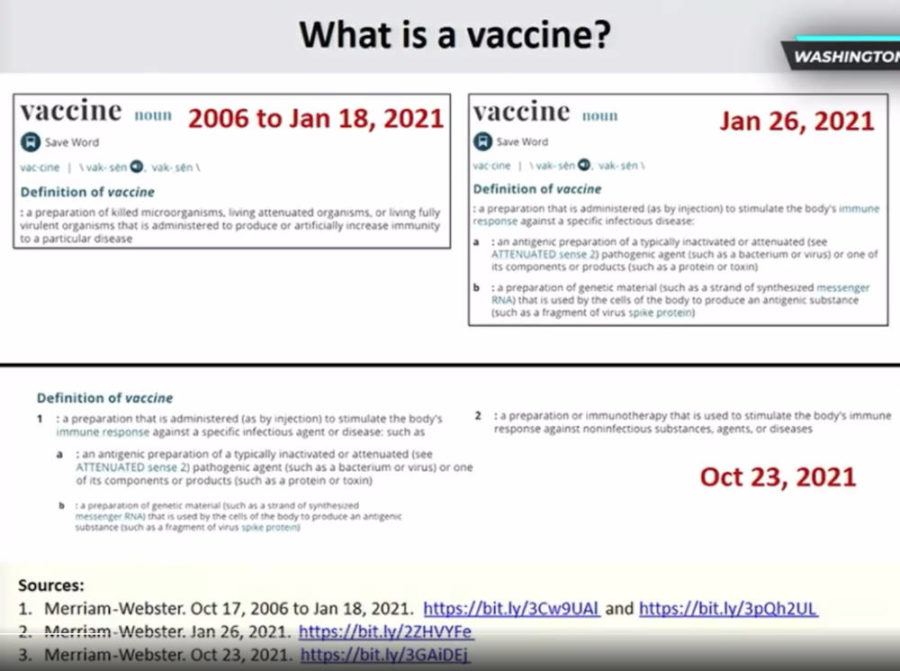
- Peter Doshi on the semantics regarding vaccines and mRNA products – Webster dictionary changed its definition of “vaccine” as mRNA products did not meet the definition! – EXCERPT
August 26, 2021 – The Highwire Episode 230: THE WRITING ON THE WALL – WATCH FDA Approves Pfizer Vaccine (Waning immunity
- The Highwire acknowledges Dr Peter Doshi’s (@39:19) recent BMJ articles – waning immunity and unblinding the clinical trial just after 2 months. Only 7% of trial participants actually reached 6 months of blinded follow up.
- They approved it looking at data prior to Delta variant, yet Delta is currently the predominant variant! They have no data for the current variant
August 23, 2021 – BMJ | Opinion: Does the FDA think these data justify the first full approval of a covid-19 vaccine? Peter Doshi – READ The elephant named “waning immunity”
- “On 28 July 2021, Pfizer and BioNTech posted updated results for their ongoing phase 3 covid-19 vaccine trial. The preprint came almost a year to the day after the historical trial commenced, and nearly four months since the companies announced vaccine efficacy estimates “up to six months.”…But you won’t find 10 month follow-up data here. While the preprint is new, the results it contains aren’t particularly up to date. In fact, the paper is based on the same data cut-off date (13 March 2021)…”
- “The 20 page preprint matters because it represents the most detailed public account of the pivotal trial data Pfizer submitted in pursuit of the world’s first “full approval” of a coronavirus vaccine from the Food and Drug Administration. It deserves careful scrutiny.”
- “Doshi noted it was highly concerning the FDA was refusing to provide transparency in their approval process or hold an open hearing on Pfizer’s vaccine prior to approving…Data after 6 months of follow-up was being deliberately withheld by Pfizer (the original trial was supposed to last for years)” – REF
The FDA should demand adequate, controlled studies with long term follow up, and make data publicly available, before granting full approval to covid-19 vaccines
Peter Doshi
June 8, 2021- BMJ Opinion: Why we petitioned the FDA to refrain from fully approving any covid-19 vaccine this year – Doshi et al – READ, Petition – PDF, ARCHIVE
- “We are part of a group of clinicians, scientists, and patient advocates who have lodged a formal “Citizen Petition” with the United States Food and Drug Administration (FDA), asking the agency to delay any consideration of a “full approval” of a covid-19 vaccine.”
May 18, 2021 – BMJ Feature | Vaccine Regulation: Covid-19 vaccines: In the rush for regulatory approval, do we need more data? Doshi – READ
- …is just six months of data from now unblinded trials acceptable… for rushed vaccine approval?
January 26, 2021 – Merriam-Webster Dictionary changes the definition of “Vaccine”, so too did the CDC – REF
January 4, 2021 – BMJ Opinion: Peter Doshi: Pfizer and Moderna’s “95% effective” vaccines—we need more details and the raw data by Peter Doshi – READ Raising MAJOR concerns on the actual efficacy for the vaccine!
- “All attention has focused on the dramatic efficacy results: Pfizer reported 170 PCR confirmed covid-19 cases, split 8 to 162 between vaccine and placebo groups. But these numbers were dwarfed by a category of disease called “suspected covid-19”—those with symptomatic covid-19 that were not PCR confirmed.”
- According to FDA’s report on Pfizer’s vaccine, there were “3410 total cases of suspected, but unconfirmed covid-19 in the overall study population, 1594 occurred in the vaccine group vs. 1816 in the placebo group.”,,With 20 times more suspected than confirmed cases, this category of disease cannot be ignored simply because there was no positive PCR test result.”
- “Competing interests: I have been pursuing the public release of vaccine trial protocols, and have co-signed open letters calling for independence and transparency in covid-19 vaccine related decision making.”
- “Doshi calculated the vaccine was likely between 19% to 29% effective and hence failed to meet the minimum standards (a 50% efficacy) for approval.“…Unfortunately, the top 5 medical journals (with the exception of the BMJ) have abjectly failed in their responsibility to inform the public of this critical information. – REF
2020
November 26, 2020 – BMJ Opinion: Pfizer and Moderna’s “95% effective” vaccines—let’s be cautious and first see the full data by Peter Doshi- READ
- Only full transparency and rigorous scrutiny of the data will allow for informed decision making, argues Peter Doshi
- “Let’s put this [efficacy] in perspective. First, a relative risk reduction is being reported, not absolute risk reduction, which appears to be less than 1%. Second, these results refer to the trials’ primary endpoint of covid-19 of essentially any severity, and importantly not the vaccine’s ability to save lives, nor the ability to prevent infection, nor the efficacy in important subgroups (e.g. frail elderly).”
October 28, 2020 – eLife: Escape from neutralizing antibodies by SARS-CoV-2 spike protein variants by Weisblum et al- READ [During clinical trial phase, before any emergency use apporval of the injectable products]
- “…shortly after Doshi’s editorial was published, a comprehensive study was published showing theSARS-CoV-2 spike protein was already evolving immunity to the antibodies directed towards it from natural immunity which raised early questions about the long term viability of pharmaceuticals directed against the original spike protein (this issue became much worse once the mRNA vaccines rapidly accelerated this trend leading to the sad situation now where we are mandating a dangerous vaccine for a virus that no longer exists).” – REF
October 21, 2020 – BMJ | Feature: Will covid-19 vaccines save lives? Current trials aren’t designed to tell us – Peter Doshi – READ, CREDIT, The Highwire Ep -188 – Watch @37min
The world has bet the farm on vaccines as the solution to the pandemic, but the trials are not focused on answering the questions many might assume they are
Peter Doshi
- “Doshi accurately described how the design of these trials fundamentally could not fulfill the public promise that the vaccines were being thoroughly evaluated for safety and efficacy” – REF
- The current phase III COVID-19 vaccine trials:
- 1. None of the trials currently under way are designed to detect a reduction in any serious outcome such as hospital admissions, use of intensive care, or deaths.
- 2. Nor are the vaccines being studied to determine whether they can interrupt transmission of the virus
- “The world has bet the farm on vaccines as the solution to the pandemic, but the trials are not focused on answering the questions many might assume they are”
- As the vaccines were failing in their initial promises, the goal posts were moved to preventing serious injury and death [bye bye stopping infection or transmission] – REF
- “Peter Hotez, dean of the National School of Tropical Medicine at Baylor College of Medicine in Houston, said, “Ideally, you want an antiviral vaccine to do two things . . . first, reduce the likelihood you will get severely ill and go to the hospital, and two, prevent infection and therefore interrupt disease transmission.”…
- Yet the current phase III trials are not actually set up to prove either. None of the trials currently under way are designed to detect a reduction in any serious outcome such as hospital admissions, use of intensive care, or deaths. Nor are the vaccines being studied to determine whether they can interrupt transmission of the virus.”
October 21, 2020 – BMJ | Editorials: Covid-19 vaccine trial protocols released – Peter Doshi – READ
- “The ongoing phase III trials for covid-19 vaccines are some of the most consequential randomised trials ever done.” Finally “four manufacturers made their full study protocols publicly available”
- The publications create a rare opportunity for “real time transparency” in which the conduct of clinical trials is opened to public scrutiny while the studies are still under way.
September 13, 2020 – NY Times: Vaccine Makers Keep Safety Details Quiet, Alarming Scientists – ARCHIVE
- ““I imagine most of the public would like to believe scientists are all sharing their data, that this process is open to scrutiny among the scientific community,” said Dr. Doshi, who has helped pressure drug makers to share trial records with researchers. [its] “Just not true.””
August 24, 2020 – BMJ | Head to Head | Correspondenct to P Doshi – Covid-19: Should doctors recommend treatments and vaccines when full data are not publicly available? – READ
February 26, 2020 – BMJ | Feature | Informed Consent : WHO’s malaria vaccine study represents a “serious breach of international ethical standards” – Peter Doshi – READ
- GIZMODO: WHO Accused of Conducting Vaccine Trial Without Participant Consent in Three African Countries – “Experts are calling it a “serious breach” of international bioethical standards and potentially “a disaster for public trust in vaccines.” – READ
- Trial slated to involve over 720,000 children in Malawi, Ghana, Africa. “Recipients of the malaria vaccine are not being informed that they are in a study,” declared Doshi in the BMJ article.
- Mosquirix, also known as the RTS,S vaccine, is the first licensed vaccine for malaria in the world”
2015
November 28, 2015 – News Punch: New Flu Shot Contains Adjuvant Linked To Gulf War Syndrome – ARCHIVE, CREDIT
- The oil-based adjuvant, known as Squalene, has been said to cause autoimmune disease, and has been linked to Gulf War Syndrome.
- Johns Hopkins Scientist Reveals Shocking [2013] Report On Flu Vaccines: Peter Doshi from the John Hopkins School of Medicine has issued a SCATHING report on the dangers of flu vaccinations in the British Medical Journal (BMJ) that completely demolishes the established ‘scientific’ theory that flu vaccinations are safe and effective.” – READ
2013
June 29, 2013 – NY Times: Breaking the Seal on Drug Research – ARCHIVE
- At aged 32, Peter Doshi “is not sure where he’ll be working come August, when his postdoctoral fellowship ends. And yet, even without a medical degree, he is one of the most influential voices in medical research today.”
- Dr. Doshi’s renown comes “from pushing the world’s biggest pharmaceutical companies to open their records to outsiders in an effort to better understand the benefits and potential harms of the drugs that billions of people take every day…. he is trying to unearth data from clinical trials — complex studies that last for years and often involve thousands of patients across many countries — and make it public.”
May 16, 2013 – News Max Health: Johns Hopkins Scientist Slams Flu Vaccine – ARCHIVE
- Doshi’s concerns echo those of Dr. Russell Blaylock, a neurosurgeon and author of “The Blaylock Wellness Report” who has deep concerns over the safety and efficacy of the flu vaccine.”
- Doshi’s article “is a breath of fresh air,” says Dr. Blaylock. “This article exposes in well-defined and articulate terms what has been known for a long time — the flu vaccine promotion is a fraud.”
May 16, 2013 – British Medical Journal (BMJ) Feature | Influenza: Influenza: marketing vaccine by marketing disease – Peter Doshi – READ, ARCHIVE, PDF, CREDIT
- The CDC pledges “To base all public health decisions on the highest quality scientific data, openly and objectively derived.” But Peter Doshi argues that in the case of influenza vaccinations and their marketing, this is not so”
- “Peter Doshi, Ph.D., says that the CDC’s policy of encouraging the public to get their yearly flu shot is based on low quality studies that fail to substantiate the claims that they prevent influenza, and that downplay the risks in taking the shots.” – REF, CREDIT
March 18, 2013 – JAMA Internal Medicine: Influenza Vaccines – Time for a Rethink – Peter Doshi – PDF, CREDIT
2012
April 10, 2012 – PLOS Medicine | Policiy Forum: The Imperative to Share Clinical Study Reports: Recommendations from the Tamiflu Experience – Doshi, Jefferson and Del Mar – READ
- “Systematic reviews of published randomized clinical trials (RCTs) are considered the gold standard source of synthesized evidence for interventions, but their conclusions are vulnerable to distortion when trial sponsors have strong interests that might benefit from suppressing or promoting selected data….”
- Unfortunately, industry and regulators have historically treated clinical study reports as confidential documents, impeding additional scrutiny by independent researchers.“
2011
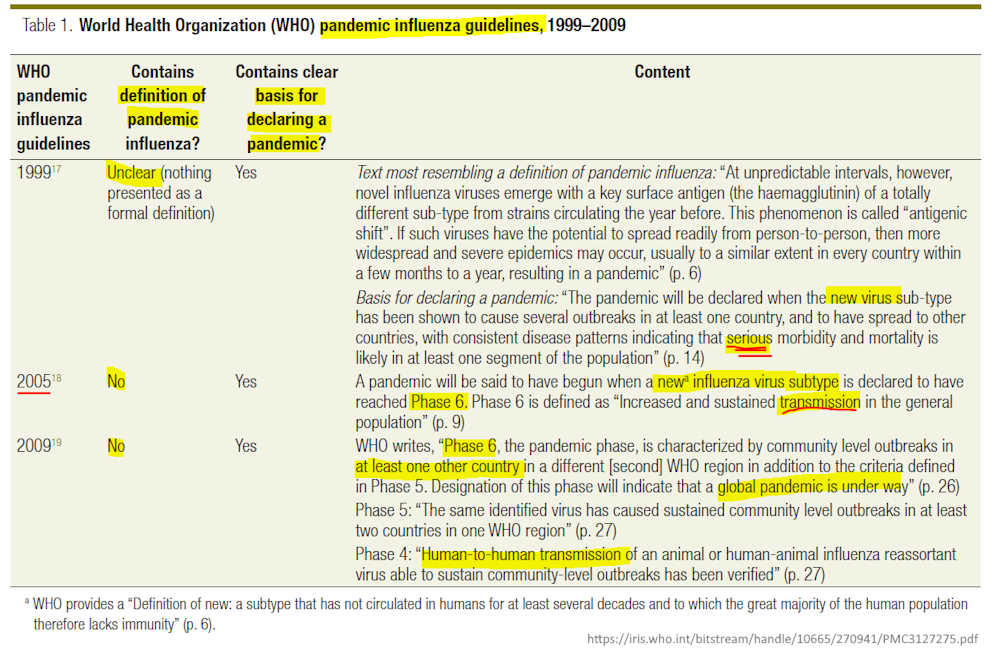
March 31, 2011 – Bulletin of the World Health Organization: The elusive definition of pandemic influenza by Peter Doshi – ARCHIVE
- “n 2009, governments throughout the world mounted large and costly responses to the H1N1 influenza outbreak. These efforts were largely justified on the premise that H1N1 influenza and seasonal influenza required different management, a premise reinforced by the decision on the part of the World Health Organization (WHO) to label the H1N1 influenza outbreak a “pandemic”….”
- “Since 2003, the top of the WHO Pandemic Preparedness homepage has contained the following statement: “An influenza pandemic occurs when a new influenza virus appears against which the human population has no immunity, resulting in several simultaneous epidemics worldwide with enormous numbers of deaths and illness.”6 However, on 4 May 2009, scarcely one month before the H1N1 pandemic was declared, the web page was altered in response to a query from a CNN reporter.7 The phrase “enormous numbers of deaths and illness” had been removed and the revised web page simply read as follows: “An influenza pandemic may occur when a new influenza virus appears against which the human population has no immunity.” Months later, the Council of Europe would cite this alteration as evidence that WHO changed its definition of pandemic influenza to enable it to declare a pandemic without having to demonstrate the intensity of the disease caused by the H1N1 virus.3“
2009
December 8, 2009 – BMJ: Neuraminidase inhibitors for preventing and treating influenza in healthy adults: systematic review and meta-analysis – Jefferson, Doshi et al – An update on 2005 Cochrane review on Tamiflu etc – READ
- The Cochrane Collaboration reported that Tamiflu could not be shown to reduce complications like pneumonia or hospitalizations. – REF
- BMJ further investigated and showed that Roche had hired ghost writers to author some of the papers involving Tamiflu…” and that those writers had said they were under pressure to highlight positive messages about the drug.”
June 2009 (Northern hemisphere Summer) – Cochrane Collaboration led by Dr. Tom Jefferson, a British epidemiologist, asked Peter Doshi to help him update review on Tamiflu – NY Times – REF
- ” IN summer 2009, Dr. Doshi received a call from Dr. Tom Jefferson, a British epidemiologist based in Rome. That year, the swine flu pandemic was spreading worldwide, and Dr. Jefferson had been hired by the British and Australian governments to update an earlier review of Tamiflu, a drug produced by the Swiss company Roche, aimed at reducing the flu’s severity and preventing more serious complications. He asked if Dr. Doshi wanted to help….
- Determining Tamiflu’s efficacy had significant economic as well as health consequences. Around the world, private companies and governments — including that of the United States — were stockpiling Tamiflu in case of influenza outbreaks, and their spending accounted for almost 60 percent of the drug’s $3 billion in sales in 2009.”
- At this time, Dr. Doshi knew little about clinical trials or even much about the drug industry. But he knew Dr. Jefferson. He met Dr. Jefferson, a prominent skeptic of the flu vaccine, after researching whether the Centers for Disease Control was exaggerating the deadliness of the disease. “We were both lone wolves in the field of influenza,”
- Jefferson assured Dr. Doshi and other researchers on his team that the update of Tamiflu “would be fairly simple….But just as their work was getting under way, a simple comment by Dr. Keiji Hayashi on the Cochrane Web questioning the efficacy of Tamiflu in children”and especially questioned the Kaiser study which Jefferson relied upon in his initial review. This “changed the course of the research and would ultimately fuel a worldwide effort to force drug companies to be more transparent” and reveal their raw data.
- Doshi soon realised on data watchdog existed. Pharma companies has simply been trusted to provide true data.
1999
1999 – World Health Organisation: – There was no formal definition of a “pandemic” – PDF, Definitions – HERE
- Pointed out in The elusive definition of pandemic influenza by Peter Doshi – CREDIT