The Oxford-AstraZeneca COVID-19 vaccine was developed and tested in partnership with The University of Oxford an the British-Swedish company AstraZeneca – a coronavirus vaccine originally known as ChAdOx1 nCoV-19 but when AstraZeneca took over it was relabled AZD1222. [1]
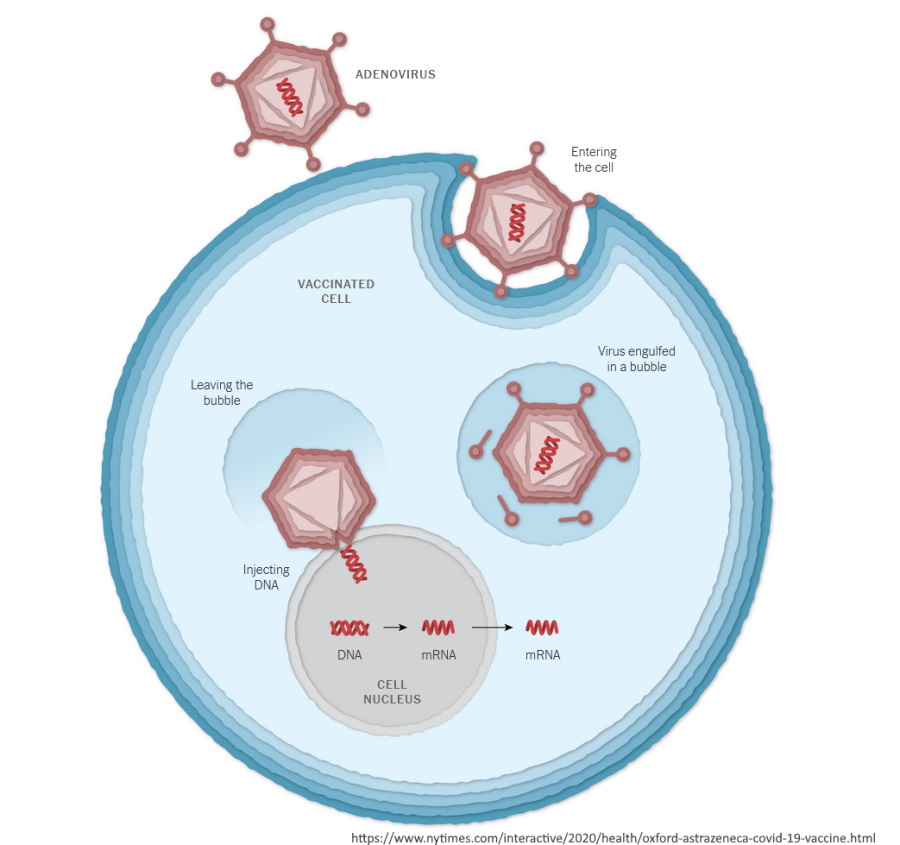
The Oxford-AstraZeneca COVID-19 vaccine is composed of a recombinant, replication-deficient, genetically modified chimpanzee adenovirus (ChAdOx1) used as a vector to transport encased DNA code.
When this vaccine is injected, the vector infects human cells and transports the DNA code into the cell nucleus. From there the body is tricked into encoding a messenger RNA (mRNA) code, which then the cytoplasm ribosomes are utilised to manufacture an S-glycoprotein of SARS-CoV-2 commonly referred to as the spike protein. The foreign protein is then expressed on the cell surface, stimulating an immune response.
The issue is the adenovirus is recognised by the body as foreign and mount an immune response to that, as well as the foreign protein the body is tricked into producing. Subsequent doses using the adenovirus would logically present a challenge, in that the bodies immune system is already primed to defend against it.
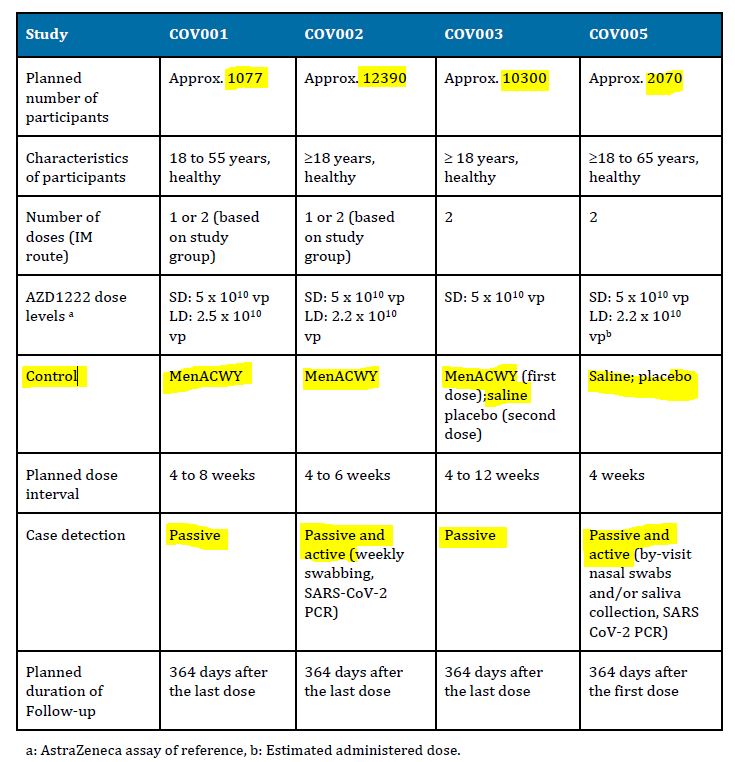
The product is designed for two full doses of the vaccine administered 4 to12 weeks apart.
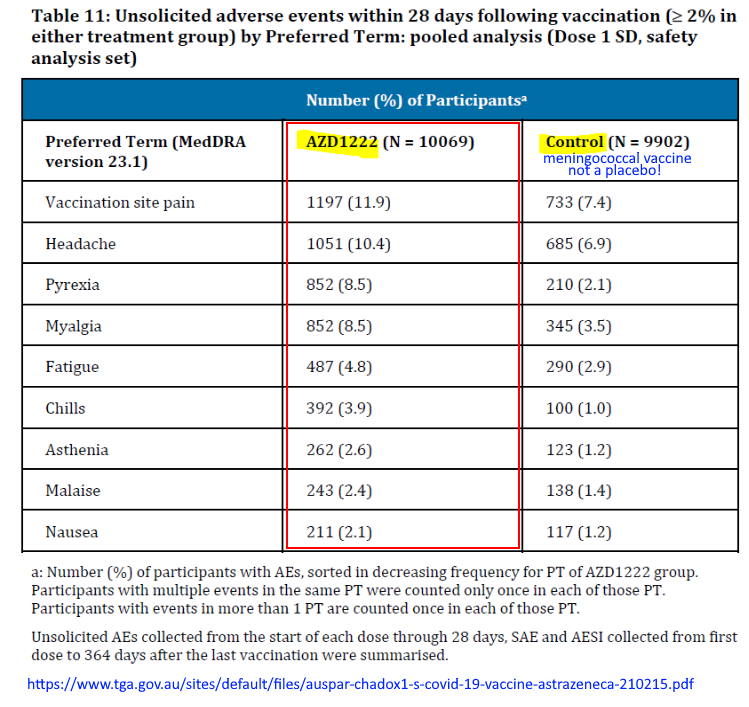
No inert placebo control were used in AstraZeneca’s trials.
Astra/Zeneca’s COVID-19 vaccine was never registered in the United States, maybe because they did not use a placebo as it’s control group in clinical trials, they used the traditional “control” – another vaccine, in this case a meningococcal vaccine – MenACWY. The justification for NOT using an inert placebo was “MenACWY was chosen as the control group vaccine to minimise the chance of accidental participant unmasking due to local or systemic reactions to the vaccine.”
AstraZeneca Website – HERE
November 5, 2020 – University of Oxford: How to make a vaccine in record time! – WATCH, ARCHIVE
AstraZeneca at Clinical Trial.gov:
- March 27, 2020 – A Study of a Candidate COVID-19 Vaccine (COV001) – Phase I/II – Uni Oxford – HERE, saline placebo!- ARCHIVE, changed placebo in April 6, 2020 to MenACWY – ARCHIVE
- May 26, 2020 – ClinicalTrials.gov: Investigating a Vaccine Against COVID-19 – Phase II/III – Uni Oxford – HERE, ARCHIVE
- August 18, 2020 – Phase III Double-blind, Placebo-controlled Study of AZD1222 for the Prevention of COVID-19 in Adults – AstraZeneca – HERE, ARCHIVE
Other Trials:
- June 23, 2020 – COVID-19 Vaccine (ChAdOx1 nCoV-19) Trial in South African Adults With and Without HIV-infection – Uni Oxford – HERE
- March 25, 2021 – A Study of Intranasal ChAdOx1 nCOV-19 – Uni Oxford – HERE
- September 29, 2021 – Safety, Efficacy of Chadox1 Ncov-19 Vaccine: Rapid Systematic Review and Meta Analysis – to study the frequency of breakthrough COVID-19 infection – Assiut University – HERE
Worrying signals began to emerge soon after the vaccine’s roll-out.
Serious blood clotting condition called thrombosis with thrombocytopenia (TTS) following vaccination, which was causing severe disability, hospitalisation and death, began to emerge soon after AstraZeneca’s COVID-19 vaccine was rollew out across the world- CREDIT
- March 7, 2021 – Reuters: Austria suspends AstraZeneca COVID-19 vaccine batch after death – READ
- March 12, 2021 – Reuters: Denmark, Norway, Iceland halt use of AstraZeneca’s vaccine – READ, ABC – READ
- March 12, 2021 – Scott Morrison stands by AstraZeneca COVID vaccine rollout after blood clot concerns overseas – READ
- March 14, 2021 – WHO says countries should keep using AstraZeneca’s COVID-19 vaccine as it investigates reports of blood clots – it hadn’t found a link between the jab and clots – READ !!!
- March 14, 2021 – AstraZeneca Press Release: Update on the safety of COVID-19 Vaccine AstraZeneca – READ
- March 17, 2021 – Business Insider: Sweden joins Germany, France, and 15 other countries in suspending AstraZeneca’s vaccine over possible side effects – READ (18 countries total)
- April 7, 2021 – Pharma Focus: AZ vax linked to clots in EU – ARCHIVE
- more links in article (I ran out of time) – READ
A/Z vaccine data points in reverse chronological order
Content is continuously being added
2024
July 9, 2024 – Maryanne Demasi Substack: The unravelling of AstraZeneca’s covid-19 vaccine – It went from being hailed as a lifesaver to getting pulled from the market. I take a look back at what happened. – READ
May 16, 2024 – The Highwire Ep 372 – ASTRAZENECA FALLOUT AMIDST VACCINE WITHDRAWAL – WATCH, FULL
May 14, 2024 – ICAN: BREAKING: Lawsuit Filed Against AstraZeneca for Mother Seriously Injured in its COVID-19 Vaccine Trial – re Brianne (“Bri”) Dressen, co-founder of REACT19 suffered severe neurological harm while participating in AstraZeneca’s U.S. COVID-19 vaccine clinical trial. – READ
- AstraZeneca is being sued for breach of contract after callously ignoring Bri’s pleas for aid. This is likely the first case of its kind…Shortly after her shot, she experienced tingling and prickling in her arms, blurred vision, headache, sound sensitivity, tinnitus, nausea, and vomiting. Bri’s condition continued to worsen as time went on, baffling her doctors, and yet AstraZeneca refused to provide any information, medical referrals, medical care, or finances to assist with her treatment.”
- The National Institutes of Health (NIH) eventually diagnosed Bri with “Post Vaccine Neuropathy”…This post-vaccine neuropathy caused chronic inflammatory demyelinating polyneuropathy (CIDP), a condition which has caused constant, abnormal, and painful sensations with which Bri has suffered, including the feeling of being repeatedly and constantly electrocuted.
- [NOTE: Prof Angus Dalgleish et al reported as early as June 2020 that the SARS-CoV-2 spike protein gene code revealed it had 100% homology with the mylin sheath – thus using it as a target antigen for a vaccine would potentially result in autoimmune issues – i.e. the body attacking it’s own nerve cells! -]
May 7, 2024 – Christine Anderson MEP: “Quietly and secretly: EU stops approval for AstraZeneca’s corona “vaccine” – GETTR
- March 27, 2024 EU AstraZeneca “withdrawing, at the holder’s request, the marketing authorisation granted by Decision C(2021) 698(final) for “Vaxzevria – COVID-19 Vaccine (ChAdOx1-S [recombinant])” effective May 7, 2024. – PDF
- This comes as they just ADMITTED in court that their product can cause severe blood clotting side effect.- READ
May 7, 2024 – The Telegraph: AstraZeneca withdrawing Covid vaccine worldwide – Company says decision is purely commercial as jab has been superseded by alternatives [yeh right!] – READ, CREDIT,
- Reuters : AstraZeneca to withdraw COVID vaccine globally as demand dips – READ
- SBS Aust: Why a popular COVID-19 vaccine has been withdrawn worldwide – READ
- ABC Aust: AstraZeneca withdraws COVID-19 vaccine citing a decline in demand – READ
- BBC: AstraZeneca to withdraw Covid vaccine – READ
April 30, 2024 – SBS: “On 30 April, AstraZeneca conceded the vaccine, sold under the name Vaxzevria, can cause fatal blood clots and low platelet counts, also known as thrombosis with thrombocytopenia syndrome, or TTS.” – REF
- “The admission came through court documents in a UK class action lawsuit which sought £100 million ($190 million) in compensation for almost 50 victims of AstraZeneca vaccine side effects.”
April 29, 2024 – Peter Sweden Substack: BOMBSHELL AstraZeneca ADMITTED side effect of covid vaccine – Massive news as the pharmaceutical giant has admitted in court that their covid vaccine can cause rare blood clotting side effect – READ
- “They are currently being sued in a class action lawsuit from victims that have suffered after being lied to by the “experts” about “safe and effective”, with lawyers saying that the AstraZeneca vaccine is DEFECTIVE…It is in this lawsuit that AstraZeneca admitted that their covid vaccine “can, in very rare cases, cause TTS“.
April 28, 2024 – The Telegraph: AstraZeneca admits its Covid vaccine can cause rare side effect in court documents for first time – Pharmaceutical giant being sued in class action over claims its vaccine caused death and serious injury in dozens of cases – READ, CREDIT
- Covid vaccine “can, in very rare cases, cause TTS” (Thrombosis with Thrombocytopenia Syndrome) that causes people to have blood clots and a low blood platelet count. – REF
- May 7, 2024 – AstraZeneca withdrew their product worldwide
February 8, 2024 – Sasha Latypova Substack: Audio recording leaked from AstraZeneca: Covid was classified a national security threat by the US Government/DOD on February 4, 2020 – READ & LISTENOn Feb 4, 2020
- AstraZeneca and other pharma companies participating in the DOD Pandemic Preparedness consortium received a phone call from the DOD saying that “novel covid virus posed national security threat”. This explains why PREP Act declaration in the US was made retroactive to Feb 4, 2020
January 18, 2024 – CHD Bus Stories: I Was In The AstraZeneca Trial – Richard started experiencing what he believes were side effects after injection how did those conducting the trial respond? – WATCH
2023
November 10, 2023 – Vaccitech (the founders of the viral vector techology) becomes Barinthus biotechnology “Guiding the immune system to cure disease” – ARCHIVE, same founders – ARCHIVE
- Pipeline “built around four proprietary platform technologies; ChAdOx, MVA, SNAP-TI and SNAP-CI.”
November 9, 2023 – The Telegraph: The real Covid jab scandal is finally emerging – The young and healthy, who were at minimal risk from Covid, should not have been told they had to take the vaccine – READ, ARCHIVE
- “Lisa Shaw died on 21 May from complications arising from the AstraZeneca Covid vaccination.”
- “The coroner said: “Ms Shaw was previously fit and well” but it was “clearly established” that her death was due to a very rare “vaccine-induced thrombotic thrombocytopenia (VITT)”, a new condition which leads to swelling and bleeding of the brain.”
- “I had lost my wife and my son had lost his mam [mum], but for an awfully long time people like us weren’t able to tell our story because we were put in the box of crackpots and conspiracy theorists,” Gareth Eve
- several leading broadcasters. “They would express sympathy, but then they were very nervous, they’d say they have to be very careful, you know, how they report the story without breaching broadcasting guidelines by implying there was any problem with the jab.” [Journalists were “guided” to keep the public in the dark]
- “15 days before Lisa Shaw went eagerly to get her Covid jab so she could “hug my mam”, Denmark stopped the use of AstraZeneca in its vaccination rollout after reports of rare but serious cases of blood clots.”
November 8, 2023 – Daily Mail: Father claims AstraZeneca’s Covid vaccine is ‘defective’ as the drugs giant faces a landmark High Court battle over the accusations – READ, News.com.au – READ, a test case with Jamie Scott and Alpa Tailor
- The Telegraph: Victims of VITT – a new condition identified by specialists – question the Government’s monitoring of the vaccine’s rollout and its efficacy – READ, ARCHIVE
- BBC “The action, taken under the Consumer Protection Act, alleges the vaccine was “defective” as it was less safe than individuals were entitled to expect.” – READ
- The Telegraph: Those injured by the AstraZeneca vaccine can no longer be silenced – ARCHIVE
- Dr John Campbell: First High Court Case – WATCH
September 15, 2022 – PRESS RELEASE: AstraZeneca Partners with Jeff Bridges, Kumail Nanjiani, and Emily V. Gordon to Educate the Immunocompromised Community about Added Protection Against COVID-19 – Up The Antibodies campaign – READ, CREDIT
- AstraZeneca launched Up The Antibodies, an important new campaign that embraces the reality for millions of immunocompromised Americans – WEBSITE, ARCHIVE
August 3, 2023 – Gen Bank: COMPOSITIONS AND METHODS FOR INDUCING AN IMMUNE RESPONSE – Gene sequence – Oxford Uni – READ, Arkmedic – CREDIT
- “The AstraZeneca vaccine, which was supposed to be different because it was a declared DNA gene therapy from the outset, had an identical amino acid sequence to the other vaccines [Pfizer, Moderna, Novavax] apart from the fact that it did not use the 2-proline mutation. Strangely its nucleotide sequence was relatively secret and only published in the patent database in August 2023″
April 6, 2023 – Dr John Campbell: AZ vaccine banned in Australia – WATCH, Dept. Health – READ, ARCHIVE

March 27, 2023 – The Telegraph: Young women had 3.5 times higher risk of death from heart issues after AstraZeneca jab – ONS found greater risk for 12 to 29 year olds in first three months after single dose of AstraZeneca Covid vaccine – READ, ARCHIVE, Dr Campbell – WATCH
March 24, 2023 – Hausfeld (UK): Claimant group brings legal claim against AstraZeneca under Consumer Protection Act 1987 – Hausfeld are acting for 41 individuals and families who have suffered devastating injuries or bereavement as a result of a rare complication of the AstraZeneca vaccine. – READ, Credit Dr Campbell – WATCH
March 24, 2023 – Dystopian Down Under Substack: High risk, low benefit of Covid boosters to healthy young people finally acknowledged by Australia’s vaccine advisory body [ATAGI] – Also, AstraZeneca has been withdrawn completely from AUSTRALIA– READ, Aust Dept of Health re AstraZeneca – READ, ARCHIVE
- “From Monday 20 March 2023 Vaxzevria (AstraZeneca) is no longer available as an approved COVID-19 vaccine” – ARCHIVE
- “A spokesperson for the Department of Health advises that in fact it was AstraZeneca who decided to discontinue the product in Australia, not the TGA, and that Vaxzevria remains provisionally approved.” – REF
2022
December 14, 2022 – Independent: Covid vaccine concerns were overblown, says AstraZeneca boss as he is knighted – Sir Pascal Soriot was speaking after he was knighted by the King at Windsor Castle on Wednesday – READ
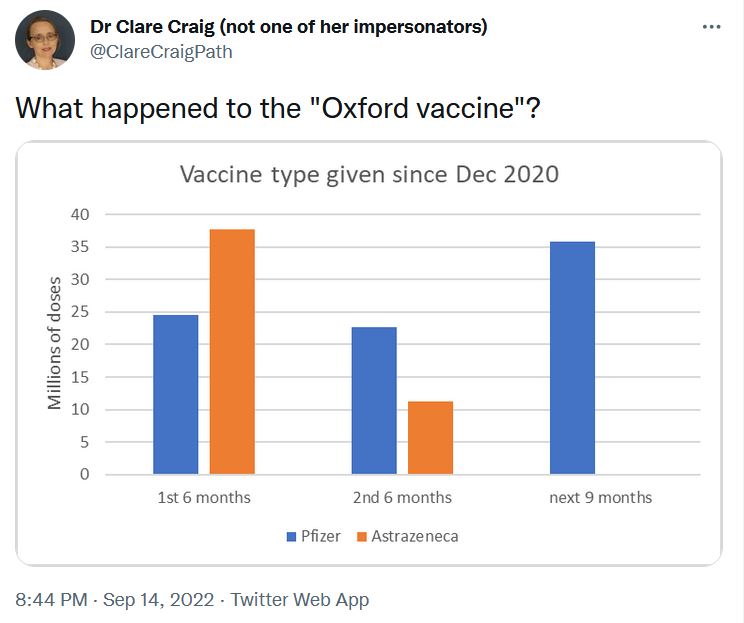
September 2022 – What happened to the “Oxford vaccine”? – TWEET “AZ has been slowly brushed under a big rug some time ago.” – TWEET
August 20, 2022 – The Naked Emperor’s Substack: Contaminated AstraZeneca batches may have caused Adverse Reactions and reduced Effectiveness – READ, July 4, 2022 – eLife: Process- and product-related impurities in the ChAdOx1 nCov-19 vaccine – Krutzke et al – STUDY
- New German study found concerning evidence of contamination of AstraZeneca vaccine batches by proteins derived from the human cell lines in which they were produced. This may have reduced the effectiveness of the vaccine by lowering the immune response. Moreover, it may have caused a variety of adverse reactions.
April 19, 2022 – Project Veritas: AstraZeneca Source Recording from 2020 Shows CEO Pascal Soriot Saying, ‘Millions of [Immunocompromised] People Can’t Be Vaccinated…Antibody [Treatment] Has Enormous Potential’ – READ, WATCH, YouTube, Daily Clout – READ
- Anonymous AstraZeneca Insider provided Project Veritas with a 2020 recording of an internal company-wide Zoom call in which CEO, Pascal Soriot, made statements about vaccine compatibility that contradicted guidance from health agencies and organizations such as the World Health Organization.
May 28, 2022 – The Telegraph: AstraZeneca vaccine may increase risk of serious neurological condition – Scientists believe the jab’s Trojan horse delivery system could be causing a rise in Guillain-Barré syndrome cases – READ, ARCHIVE
February 8, 2022 – TGA approves provisional registration for VAXZEVRIA (previously COVID-19 Vaccine AstraZeneca) COVID-19 booster dose for individuals aged 18 years and over – READ, name change – HERE
2021
December 2, 2021 – The Telegraph: Scientists discover ‘smoking gun’ link between AstraZeneca vaccine and lethal blood clots – Researchers finally get to the bottom of the biological process that has led to 73 deaths out of 50m doses of Covid jab administered in UK – READ
- The new studyfrom Cardiff University discovered that adenovirus vector attracts a blood protein called “platelet factor four”.
- [This is curious since in Australia the OGTR – “assessed” the vector , but not the DNA inside, concluded “The risk assessment concluded that risks to the health and safety of people or the environment from the proposed supply, either in the short or long term, are negligible” – PDF]
December 2, 2021 – The Telegraph: Astrazeneca Covid vaccine Q&A: What we know about blood clots, its trigger, the side effects and risks – The UK medicines regulator says the benefits of the AstraZeneca vaccine continue to outweigh any risks – ARCHIVE
- In June 2021, regulators globally concluded that “very rare cases of a particular type of blood clot may be linked to the AstraZeneca/Oxford University vaccine.”
- April 2021 Oxford University study [the creators of the vaccine!!] determined “the risk of the rare clot from the vaccine is eight times less than the risk of a clot caused by Covid-19”!
- “…blood clots led to 73 deaths out of 50m administered doses of the Covid jab,” – under 40’s no longer offered May 2021
October 14, 2021 – CSL: CSL Reaffirms Commitment to Manufacture AstraZeneca COVID Vaccine into 2022 – CSL and Seqirus- READ, ARCHIVE
August 24, 2021 – Australian Academy of Science: AstraZeneca vaccine: risk of death is 1 in a million, but what does that mean? – READ, ARCHIVE …and “that it was more likely you’d be struck by lightning, than die from TTS”. CREDIT
- “The risk of being struck by lightning in any given year is 1 in 500,000, based on data from the CDC in the United States of America (2013), accessed 9 August 2021″
July 21, 2021 – ATAGI ssesses AstraZeneca COVID-19 vaccine and Thrombosis and Thrombocytopenia Syndrome (TTS) cases in Australia. – READ
July 22, 2021 – The New Daily: Two more Australian linked to AstraZeneca shot – They were a 44-year-old Tasmanian man and a 48-year-old woman from Victoria. – READ, OTHER, Yahoo NEWS
- “The two new confirmed TTS deaths take the total to five in Australia from more than 6.1 million AstraZeneca doses. They include four women, two aged 48, and the others aged 52 and 72. The other death is the 44-year-old man. All are linked to people having their first dose.”…
- “Pfizer remains the recommended Covid-19 vaccine for Australians under 60 due to the extremely rare AstraZeneca-related blood clotting condition being more prevalent in younger people.”
“It is one in a million,” emphasing the vaccine’s low mortality rate
“One is too many, but what we are trying to achieve is a balance between that rare but serious side effect and the absolute fundamental good that is vaccinating our community against Covid-19.”
Victoria’s acting chief health officer Ben Cowie
July 14, 2021 – CHD | The Defender – Brianne Dressen, who accumulated more than $250,000 in medical bills after participating in AstraZeneca’s COVID vaccine clinical trial, is collaborating with two U.S. Senators to get help for others injured by COVID vaccines. – READ
July 7, 2021 – ATAGI update following weekly COVID-19 meeting – READ
- ATAGI met to review the latest developments relating to the AstraZeneca COVID-19 vaccine and Thrombosis and Thrombocytopenia Syndrome (TTS) cases in Australia.
July 1, 2021 – MSN: Ian’s 34yo daughter Katie Lees died after receiving AstraZeneca’s COVID-19 vaccine. He wants to honour her. But he also wants answers – READ
May 7, 2021 – BBC: Under 40s to be offered alternative to AZ vaccine – due to a link with rare blood clots – READ
- The UK’s medicines safety regulator says there have been 242 clotting cases and 49 deaths, with 28.5 million doses of the vaccine administered. But the risk is slightly higher in younger age groups.
May 6, 2021 – The Sun: PEOPLE under 40 will be offered an alternative to the Oxford/AstraZeneca jab due to blood clot fears. – READ, The Telegraph UK – READ
- Officials said the move was “precautionary” and that the risk of a blood clot is still “extremely small”.
- 242 cases of clotting identified, including 49 deaths. – REF
April 30, 2021 – The UK Telegraph: AstraZeneca chief rejects EU accusations of ‘overpromising’ vaccine supply to bloc – READ
- A/Z delivered only a quarter of EU commitment by March end. Plans 100 million doses by the end of June, “far below the 300 million foreseen in the contract.”
April 23, 2021 – TGA: AstraZeneca ChAdOx1-S COVID-19 vaccine – Three additional Australian cases of thrombosis with thrombocytopenia syndrome (TTS) likely linked to vaccine – ARCHIVE, more TGA media release – HERE
- “The Therapeutic Goods Administration (TGA) convened a Vaccine Safety Investigation Group (VSIG) meeting to review three newly reported cases of suspected thrombosis with thrombocytopenia syndrome (TTS) following vaccination with the AstraZeneca COVID-19 vaccine. The cases were in a 35 year old NSW woman, a 49 year old QLD man and an 80 year old Victorian man. Symptom onset ranged from 9 to 26 days after vaccination. TTS is a rare specific syndrome that occurs when a person has blood clots (thrombosis) as well as low blood platelet counts (thrombocytopenia)”
- April 16, 2021 – Third Australian case of thrombosis with thrombocytopenia likely linked to vaccine – ARCHIVE
- April 13, 2021 – Second case of thrombosis with thrombocytopenia – ARCHIVE
- April 9, 2021 – Updated safety advisory – rare and unusual blood clotting syndrome (thrombosis with thrombocytopenia) – ARCHIVE
- April 2, 2021 – Specific clotting condition reported after COVID-19 vaccination – Some rare cases of thrombosis associated with thrombocytopenia have been reported overseas following the administration of COVID-19 Vaccine AstraZeneca.- ARCHIVE
- TGA is working closely with the Australian Technical Advisory Group on Immunisation (ATAGI) and expert haematologists on the investigation of case reports…TGA’s assessment is that the benefits of vaccination against COVID-19 continue to outweigh any risks
- April 2, 2021 – Dept Health Aust: Updated ATAGI statement for healthcare providers on a specific clotting condition being reported after COVID-19 vaccination –ARCHIVE
- The investigations in Europe and the UK are looking at unusual cases of thrombosis (predominantly CVST) with occurs with thrombocytopenia. Investigators have not confirmed a causal link with the AstraZeneca COVID-19 vaccine. However, the investigation is ongoing.”
- April 2, 2021 – Statement by Acting Australian Government Chief Medical Officer, Professor Michael Kidd and Head of the Therapeutic Goods Administration Adjunct Professor John Skerritt – READ
- Australia’s vaccine safety and regulatory process is world class and people can be confident that vaccines approved for use are safe and effective. Our vaccines will save lives and are an essential part of tackling this global pandemic. Australia is taking a phased approach to the vaccination program roll out. Australia is currently utilising two vaccines – the Pfizer and the AstraZeneca vaccines.” BUT “A small number of people, predominantly overseas (in the United Kingdom and Europe) have presented with clotting disorders following vaccination with the AstraZeneca vaccine.”
April 23, 2021 – The UK Telegraph: ‘Benefits outweigh the risks'[of “rare” blood clots]: New data shows why European regulators back AstraZeneca jab – for all ages …- READ
April 21, 2021 – AUSTRALIA: “…the Northern Daily Leader reported [no archive] that a man in the northern NSW city of Tamworth had died in hospital on 21 April from blood clots in his lungs, which developed after he received the vaccine. And the ABC reported that a man in his 70s had died in Sydney after receiving the vaccine, but did not name the cause of the man’s death”. AstraZeneca being investigated – SOURCE
April 16, 2021 – TGA: Third Australian case of thrombosis with thrombocytopenia likely linked to vaccine – AstraZeneca – READ, ARCHIVE, SOURCE
- On April 8, 2021 a 48-year-old woman in NSW who was vaccinated on 8 April was “likely to be linked to vaccination”. She was “admitted to hospital with an extensive thromboembolic event and thrombocytopenia (TTS) four days after receiving the AstraZeneca COVID-19 Vaccine.” She died in hospital.
- April 8, 2021 – Daily Mail: Woman, 61, becomes the third Australian to die after taking the AstraZeneca coronavirus vaccine with her extremely rare death ‘likely linked’ to the jab – but she had a pre-existing condition and you’ve got more chance of being struck by lightning – READ
April 8, 2021 – ABC News Aust: AstraZeneca blood clot concern sees Australian government name Pfizer as preferred vaccine for adults under 50 – READ
- Australian health authorities have advised the Pfizer vaccine should be given to Australians aged under 50, amid concerns of rare blood clots potentially linked to the AstraZeneca vaccination.
April 8, 2021 – Dept Health ATAGI statement on AstraZeneca vaccine in response to new vaccine safety concerns – READ
April 7, 2021 – BBC: Under-30s in the UK are to be offered an alternative Covid vaccine to the AstraZeneca jab due to the evidence linking it to rare blood clots. – READ
- The recommendation comes after a review by by drugs regulator MHRA found by the end of March 79 people in the UK had suffered rare blood clots after vaccination – 19 of whom had died. – READ
April 2, 2021 – Aust Dept Health: Statement by Acting Australian Government Chief Medical Officer, Professor Michael Kidd and Head of the Therapeutic Goods Administration Adjunct Professor John Skerritt – ARCHIVE, more TGA media release – SOURCE
- A small number of people, predominantly overseas (in the United Kingdom and Europe) have presented with clotting disorders following vaccination with the AstraZeneca vaccine.
April 1, 2021 – NY Post: US may not even need AstraZeneca COVID-19 vaccine, Fauci says – READ
March 31, 2021 – CIDRAP: AstraZeneca COVID vaccine 70% effective vs B117 variant – READ, STUDY, (B.1.1.1 is Alpha variant)
March 31, 2021 – NY Post: Woman suffers agonizing rash after Oxford-AstraZeneca COVID vaccine – READ
March 26 2021 – Pharma in focus: AZ hire puts shine back into vax – – ARCHIVE, CREDIT
- Plagued by poor publicity over efficacy, blood clots and outdated data, the AstraZeneca COVID-19 vaccine is about to get a boost with the company creating a new role in Australia to build medical confidence in the jab, on the cusp of a mass nationwide rollout – by hiring a Vaccine Medical Communications Specialist
March 25, 2021 – NY Post: AstraZeneca releases revised COVID-19 vaccine data following rift with US officials – “company says shows a 76 percent effectiveness rate in preventing symptomatic cases of the coronavirus.” – READ
March 23, 2021 – TGA: Melbourne-manufactured AstraZeneca vaccine is now available for Australians – CSL-Seqirus – ARCHIVE
- TGA) approved ” the release of the first four batches (batches 300157P, 300158P, 300160P and 300161P) totalling 832,200 doses for supply”.
- “TGA testing of the vaccine batches in our Canberra laboratories plus review of extensive manufacturing documentation, has ensured that the locally-manufactured vaccine has the exactly the same composition and performance as the overseas-manufactured vaccine, the same quality, and is free of contaminants.”
- TGA approval is required for each and every batch of any vaccine supplied in Australia.
- March 21, 2024 – TGA approves CSL – Seqirus to manufacture AstraZeneca COVID-19 vaccine in Australia – ARCHIVE
- This follows on from the 16 February 2021 approval by TGA of the overseas-manufactured AstraZeneca vaccine.
- The vaccine is being manufactured at two sites in suburban Melbourne. CSL-Behring Australia in Broadmeadows are manufacturing the active raw vaccine material, while the final vaccine doses are being manufactured, vials filled and packaged at Seqirus in Parkville (a CSL company).
- The Australian Government has purchased 50 million doses of the AstraZeneca vaccine, to be manufactured on their behalf by CSL
March 23, 2021 – NIH Press Release: NIAID Statement on AstraZeneca Vaccine – READ, CREDIT
- Data and Safety Monitoring Board (DSMB) notified NIAID, BARDA, and AstraZeneca that it was concerned by information released by AstraZeneca on initial data from its COVID-19 vaccine clinical trial.
“It really is unfortunate that this happened,” … “The fact is, this is very likely a very good vaccine, and this kind of thing does … really cast some doubt about the vaccines and maybe contribute to the hesitancy. It was not necessary.”
Dr Anthony Fauci told Good Morning America
March 22, 2021 – BMJ: Covid-19: AstraZeneca vaccine prevents 79% of symptomatic disease and 100% of severe disease, US study finds – READ
March 22, 2021 – Forbes: AstraZeneca Vaccine 100% Effective In Preventing Severe Disease And Hospitalizations, U.S. Trials Show – READ
March 22, 2021 – NY Post: AstraZeneca’s COVID-19 vaccine found to be 79 percent effective: study – READ
- Although AstraZeneca’s vaccine has been authorized in more than 50 countries, it has not yet been given the green light in the U.S. The U.S. study comprised more than 30,000 volunteers,”
March 22, 2021 – AstraZeneca Press Release: AZD1222 US Phase III trial met primary efficacy endpoint in preventing COVID-19 at interim analysis – READ, ARCHIVE
- “This interim safety and efficacy analysis was based on 32,449 participants accruing 141 symptomatic cases of COVID-19. The trial had a 2:1 randomisation of vaccine to placebo.” [“placebo” is not inert, its another vaccine!]
- 79% vaccine efficacy at preventing symptomatic COVID-19
- 100% efficacy against severe or critical disease and hospitalisation
- “The US Phase III trial, called D8110C00001, was led by AstraZeneca and funded by the Biomedical Advanced Research and Development Authority (BARDA), part of the office of the Assistant Secretary for Preparedness and Response (ASPR) at the US Department of Health and Human Services (HHS) in collaboration with the Department of Defense Joint Program Executive Office for Chemical, Biological, Radiological and Nuclear Defense (JPEO-CBRND) and the Army Contracting Command, and the National Institute of Allergy and Infectious Diseases (NIAID), part of the US National Institutes of Health. The NIAID-supported COVID-19 Prevention Network (CoVPN) participated in the trial.” Clinical trial NCT04516746 – REF
March 19, 2021 – NY Post: Four European countries hold off on AstraZeneca COVID vaccinations – Denmark, Norway and Sweden said they would wait until next week to decide whether to resume their rollouts of the shot after several people who received it developed blood clots. Finland halted its use – READ, Finland resumes use – READ
March 19, 2021 – TGA Press Release : AstraZeneca ChAdOx1-S COVID-19 vaccine Update – European and UK reviews find no proven link with blood clots – ARCHVIE
March 19, 2021 – TGA Press Release : AstraZeneca ChAdOx1-S COVID-19 vaccine Update – Expert review finds no evidence of increased risk of anaphylaxis – ARCHIVE, SOURCE
March 17, 2021 – Business Insider: Sweden joins Germany, France, and 15 other countries in suspending AstraZeneca’s vaccine over possible side effects – READ (18 countries total)
March 16, 2021 – Aust Dept Health: Therapeutic Goods Administration Adj. Professor John Skerritt’s interview on 2GB Radio on 16 March 2021 – READ, ARCHIVE, CREDIT
“We don’t have any evidence that there is an effect, but we’re working very closely with the Europeans on this”.
…With the information we have at the moment, we don’t have evidence that would justify pausing the roll out [in Australia] because we don’t have evidence that this is an effect of the vaccine…And so, until we have evidence that there’s cause and effect, we won’t be pausing the rollout.
…And similarly, the British, who have more experience than anyone else in the world – some 11 million people have been vaccinated in the UK with the vaccine – have not found any evidence of these clots….A bit like hamburgers, there’s an awful lot of hamburgers sold, and the British have not found any problems.
Adj. Professor John Skerritt, head of TGA
- Chief Medical Officer, Professor Paul Kelly’s press conference on 16 March 2021 – “no evidence that it causes blood clots” – READ
- March 12, 2021 – Scott Morrison stands by AstraZeneca COVID vaccine rollout after blood clot concerns overseas – READ
March 14, 2021 – WHO says countries should keep using AstraZeneca’s COVID-19 vaccine as it investigates reports of blood clots – it hadn’t found a link between the jab and clots – READ !!!
March 14, 2021 – AstraZeneca Press Release: Update on the safety of COVID-19 Vaccine AstraZeneca – READ
March 11, 2021 – NY Post: Denmark, Norway and Iceland halt AstraZeneca COVID vaccine over blood clots – READ, Denmark halt – READ, Reuters – READ
March 10, 2021 – European Medicines Agency: COVID-19 Vaccine AstraZeneca: PRAC preliminary view suggests no specific issue with batch used in Austria – ” a person was diagnosed with multiple thrombosis (formation of blood clots within blood vessels) and died 10 days after vaccination,” – READ
March 7, 2021 – Reuters: Austria suspends AstraZeneca COVID-19 vaccine batch after death – READ
March 7, 2021 – Australian Minister for Health and Aged Care The Hon Greg Hunt MP Media Release: Second COVID-19 vaccine now in use in Australia – more doses of the University of Oxford-AstraZeneca vaccine being provided to Australians this week – READ, ARCHIVE
- “On 3 February 2021 the Lancet Journal said, “COVID-19 Vaccine AstraZeneca confirms 100% protection against severe disease, hospitalisation and death in the primary analysis of Phase III trials.”” stated Greg Hunt
March 1, 2021 – The Highwire: COVID VAX NEWEST GUINEA PIGS- “Covid-19 vaccine testing is now expanding to children as young as six in AstraZeneca trials in the UK- WATCH
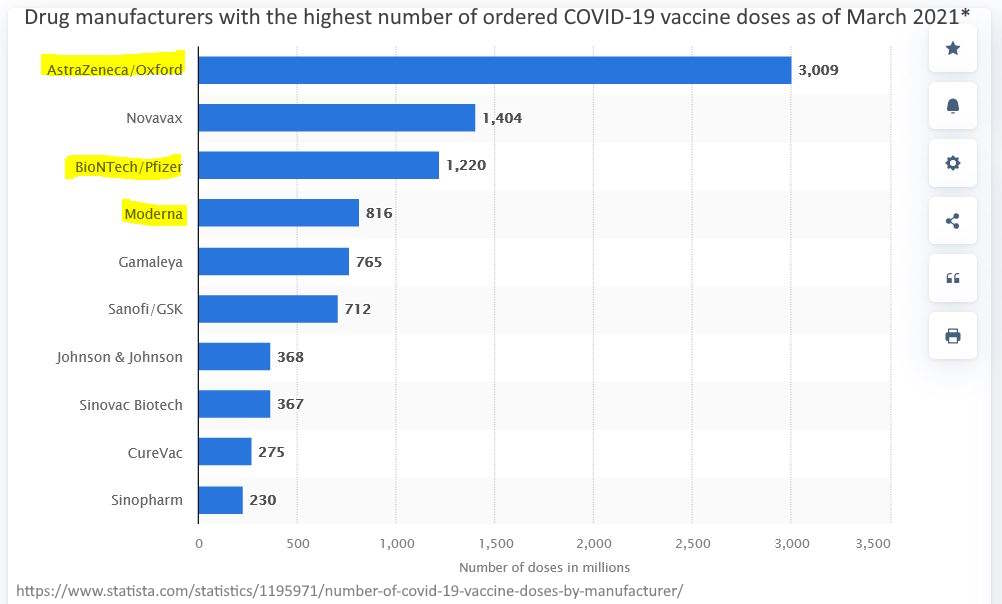
March 2021 – Statista: Drug manufacturers with the highest number of ordered COVID-19 vaccine doses as of March 2021* – READ
Febraury 16, 2021 – Prime Minister Scott Morrison Press Release: TGA approves AstraZeneca COVID-19 vaccine – ARCHIVE, Facebook – WATCH
- “The Therapeutic Goods Administration (TGA) has today approved the AstraZeneca COVID-19 vaccine for use in Australia following a full and thorough assessment process. The vaccine has met the required standards for safety, quality and efficacy and will be provided free to Australians.” [so misleading it’s “provisional” standards]
- [And at the very end of the presser] “Every safety and regulatory box has been ticked for provisional approval – and further ones will now be ticked in the lead up to March.” [Its techically “provisional registration” not approval]
- “Initial supply into Australia will be imported from overseas. In the coming months, the AstraZeneca vaccine will be manufactured in Australia. Australia is one of a small number of countries in the world that can manufacture its own COVID-19 vaccine.”…”The Australian Government has secured 53.8 million doses of the AstraZeneca vaccine, with 50 million doses being manufactured here in Australia.”
- “The AstraZeneca COVID-19 vaccine is provisionally approved in Australia for active immunisation to prevent coronavirus disease 2019 (COVID-19), caused by SARS-CoV-2, in individuals 18 years of age and older.”
- The Australian Technical Advisory Group on Immunisation (ATAGI) determined that the 2 doses should be administered 12 weeks apart. “However, if this preferred interval is not possible, for example because of imminent travel, cancer chemotherapy, or major elective surgery, a minimum interval of 4 weeks between doses can be used.”
- “Data from clinical trials tells us that the AstraZeneca vaccine will stop people becoming seriously unwell with COVID-19. This is – and must – be our first priority. …” Minister Hunt said. “The global evidence is of overwhelming protection against serious illness, hospitalisation and loss of life.” [these comments support that “active immunisation” is against symptoms not preventing infection]
- WHO’s statement reinforces “preventing symptomatic COVID-19”
- Australian Government: Coronavirus (COVID-19) The latest official coronavirus news, updates and advice from the Australian Government – Feb 2021 – ARCHIVE
February 16, 2021 – TGA Press Release: TGA provisionally approves AstraZeneca’s COVID-19 vaccine – ARCHIVE, Label information – HERE
- making it the second COVID-19 vaccine to receive regulatory approval in Australia.
- “The decision to provisionally approve the vaccine was also informed by expert advice from the Advisory Committee on Vaccines (ACV), an independent committee with expertise in scientific, medical and clinical fields including consumer representation.” [who have conflicts of interest – Allen Cheng]
February 15, 2021 – Australia’s TGA grant provisional registration for AstraZeneca ChAdOx1-S COVID-19 vaccine for 18 years and over – READ, TGA PRESS
- “Following a thorough and independent review, the TGA has decided that the following vaccines meet the high safety, efficacy and quality standards required for use in Australia.”
February 8, 2021 – Office of the Gene Technology Regulator (OGTR) Australia: Summary of the Risk Assessment and Risk Management Plan for Licence Application DIR 180 – PDF, License to supply – PDF, Gene Technology Technical Advisory Committee (GTTAC) meeting – READ
- “… a licence for application (DIR 180) for the import, transport, storage and disposal of a genetically modified (GM) COVID-19 vaccine, as part of its commercial supply as a human vaccine.”
- “The risk assessment concluded that risks to the health and safety of people or the environment from the proposed supply, either in the short or long term, are negligible. The current assessment focused on risks posed to people other than the intended vaccine recipient and to the environment,…” [TGA to assess recipient safety!]
- [Curously ONLY the virus vector is assessed by this “gene regulator” agency NOT the gene inside!!! mRNA vaccines are 100% exempt! – ONLY GMO’s are assessed, not genes! – extremely deceptive]
February 3, 2021 – AstraZeneca Press Release: COVID-19 Vaccine AstraZeneca confirms 100% protection against severe disease, hospitalisation and death in the primary analysis of Phase III trials – READ, ARCHIVE
- “The primary analysis for efficacy was based on 17,177 participants accruing 332 symptomatic cases from the Phase III UK (COV002), Brazil (COV003) and South Africa (COV005) trials led by Oxford University and AstraZeneca, a further 201 cases than previously reported.”
- “The primary analysis of the Phase III clinical trials from the UK, Brazil and South Africa, published as a preprint in The Lancet confirmed COVID-19 Vaccine AstraZeneca is safe and effective at preventing COVID-19, with no severe cases and no hospitalisations, more than 22 days after the first dose.”
- “Resultsdemonstrated vaccine efficacy of 76% (CI: 59% to 86%) after a first dose, with protection maintained to the second dose. With an inter-dose interval of 12 weeks or more, vaccine efficacy increased to 82% (CI: 63%, 92%).”
- “The analysis also showed the potential for the vaccine to reduce asymptomatic transmission of the virus, based on weekly swabs obtained from volunteers in the UK trial. The data showed that PCR positive readings were reduced by 67% (CI: 49%, 78%) after a single dose, and 50% (CI: 38% to 59%) after the two dose regimen, supporting a substantial impact on transmission of the virus.”
- Feb 1, 2021- Lancet (preprint): Single Dose Administration, And The Influence Of The Timing Of The Booster Dose On Immunogenicity and Efficacy Of ChAdOx1 nCoV-19 (AZD1222) Vaccine – READ
January 28, 2021 – Express UK: Germany to stop giving AstraZeneca jab to over-65s in bombshell move – the Standing Vaccine Commission at the Robert Koch Institute, Germany’s main public health agency, said: “There are currently insufficient data available to assess the vaccine efficacy from 65 years of age – READ
January 4, 2021 – The Guardian: Oxford man, 82, first in world to get Oxford/AstraZeneca jab – Dialysis patient Brian Pinker – READ, ARCHIVE, BBC – READ
2020
December 30, 2020 – UK regulator MHRA approves the Oxford-AstraZeneca COVID-19 vaccine for use in Britain. READ, SOURCE
December 8, 2020 – The Lancet: Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK – READ
- “Data published in The Lancet on December 8 show the vaccine was 62% effective in preventing COVID-19 in a group of 4,440 people given two standard doses, yet offered 90% protection in a subgroup of 1,367 people who were given a first half dose followed by a full second dose.” – SOURCE
November 27, 2020: Bloomberg: Astra Eyes Extra Global Vaccine Trial as Questions Mount – READ, Forbes: AstraZeneca May Conduct Additional Trial For Covid-19 Vaccine After Acknowledging Errors In Initial Trial – READ
- “In an interview with Bloomberg, AstraZeneca CEO Pascal Soriot said that the company has now found what looks like a more effective dose and it has to validate this, “so we need to do an additional study,” which would be outside the U.S. and faster since it would need a smaller number of patients.” – REF
- “Compared to Pfizer and Moderna’s mRNA vaccines AstraZeneca’s shot is easier and cheaper to manufacture, transport, and store, which means it is more likely to be used around the world.”
- “the botched trial data have led to concerns on whether the vaccine will be cleared by regulators. Moncef Slaoui, the head of the U.S. government’s Operation Warp Speed vaccine program first disclosed that the lower dose trial was administered to some people because of an error in the quantity of vaccine put into some vials and he added that this arm of the trial was conducted on a younger population, both of which the company had failed to disclose in its announcement on Monday.”
November 26, 2020 – Bloomberg: AstraZeneca Faces More Vaccine Questions After Manufacturing Error – READ
- “…the botched trial data have led to concerns on whether the vaccine will be cleared by regulators. Moncef Slaoui, the head of the U.S. government’s Operation Warp Speed vaccine program first disclosed [via Bloomberg] that the lower dose trial was administered to some people because of an error in the quantity of vaccine put into some vials and he added that this arm of the trial was conducted on a younger population, both of which the company had failed to disclose in its announcement on Monday [23rd]”
November 24, 2020 – Market Watch: AstraZeneca’s COVID-19 vaccine data fails to impress Wall Street analysts with its effectiveness in clinical trials – SVB Leerink’s Geoffrey Porges is not expecting AstraZeneca’s COVID-19 vaccine candidate to be licensed in the U.S. – READ, [It never was use in the US]
November 23, 2020 – Forbes: AstraZeneca-Oxford Vaccine Up To 90% Effective At Preventing Covid-19, Early Results Show – READ, Bloomberg: Astra-Oxford Vaccine Found Effective in Preventing Covid – READ, CBS NEWS – WATCH,
- “Pharmaceutical giant AstraZeneca is preparing to submit its Covid-19 vaccine, developed in conjunction with the University of Oxford, to regulatory authorities for emergency approval after a preliminary analysis of an ongoing clinical trial revealed it to be up to 90% effective at preventing Covid-19. — while the findings follow 95% success rates from Moderna and Pfizer’s vaccines, the Oxford vaccine is cheaper and much easier to store and transport. “
- STAT News: AstraZeneca Covid-19 vaccine is 70% effective on average, early data show – READ
- “Two full doses of the vaccine appeared to be only 62% effective at preventing disease, while a half dose, followed by a full dose [n = 2,741], was about 90% effective.”
November 23, 2020 – Market Watch: AstraZeneca–Oxford COVID-19 vaccine can be up to 90% effective, late-stage trials show – ARCHIVE
- AstraZeneca’s vaccine candidate can be stored at normal refrigeration temperatures, not needing the supercool storage the Pfizer vaccine requires
November 23, 2020 – PRESS RELEASE: Oxford University breakthrough on global COVID-19 vaccine – READ
- “These preliminary data indicate that the vaccine is 70.4% effective, with tests on two different dose regimes showing that the vaccine was 90% effective if administered at a half dose and then at a full dose, or 62% effective if administered in two full doses.”
These findings show that we have an effective vaccine that will save many lives…
Professor Andrew Pollard, Director of the Oxford Vaccine Group and Chief Investigator of the Oxford Vaccine Trial
The announcement today takes us another step closer to the time when we can use vaccines to bring an end to the devastation caused by SARS-CoV-2.
Professor Sarah Gilbert, Professor of Vaccinology at the University of Oxford
November 18, 2020 – The Lancet (preprint): Safety and immunogenicity of ChAdOx1 nCoV-19 vaccine administered in a prime-boost regimen in young and old adults (COV002): a single-blind, randomised, controlled, phase 2/3 trial – READ
October 9, 2020 – PRESS RELEAE: COVID-19 Long-Acting AntiBody (LAAB) combination AZD7442 rapidly advances into Phase III clinical trials – READ, CREDIT
- AstraZeneca’s long-acting antibody (LAAB) combination, AZD7442, will advance into two Phase III clinical trials in more than 6,000 participants at sites in and outside the US that are due to begin in the next weeks… LAABs have been engineered with AstraZeneca’s proprietary half-life extension technology to increase the durability of the therapy for six to 12 months following a single administration.
- A/Z received $486m from US Govt for for the development and supply of AZD7442 under an agreement with BARDA and DOD
And for those of you who may not be totally familiar with antibodies, you know, you have to know a number of people cannot be vaccinated, like if you have an immune disease, lupus or some other immune condition… or multiple sclerosis, you cannot be vaccinated. So, there are millions of people in the world that will need the protection that cannot be coming from a vaccine, so the long-acting antibody has the enormous potential.
CEO of AstraZeneca, Pascal Soirot, Feb 4, 2020
September 29, 2020 – The Highwire: COVID VACCINE TECHNOLOGY’S DANGEROUS PAST- “AstraZeneca’s COVID-19 viral vector vaccine technology is under increased scrutiny after two reports of transverse myelitis in trial groups. Previously, viral vector technology led to the death of Jesse Gelsinger, who died after being treated with a modified virus in a gene therapy trial”. – WATCH
August 18, 2020 – Clinical Trial gov: Phase III Double-blind, Placebo-controlled Study of AZD1222 for the Prevention of COVID-19 in Adults – Identifier: NCT04516746 – ARCHIVE, READ Other Study ID Numbers: D8110C00001
- The aim of the study is to assess the safety, efficacy, and immunogenicity of AZD1222 for the prevention of COVID-19. Trial proposed 20,000 treatment group and 10,000 saline placebo
- Sponsored by AstraZeneca, Collaborator: Iqvia Pty Ltd (Australia/NZ) – WEB ARCHIVE
- D8110C00001 trial – led by AstraZeneca, funded by BARDA, part of ASPR at HHS, in collaboration with DOD JPEO-CBRND and the Army Contracting Command and NIAIDs CoVPN – REF
July 31, 2020 – The Highwire: DEEP DIVE INTO THE COVID VACCINE TRIAL – The HighWire looks inside the Lancet’s latest study data on Astra Zeneca’s vaccine being tested at Oxford University, “the numbers are shocking”. – WATCH
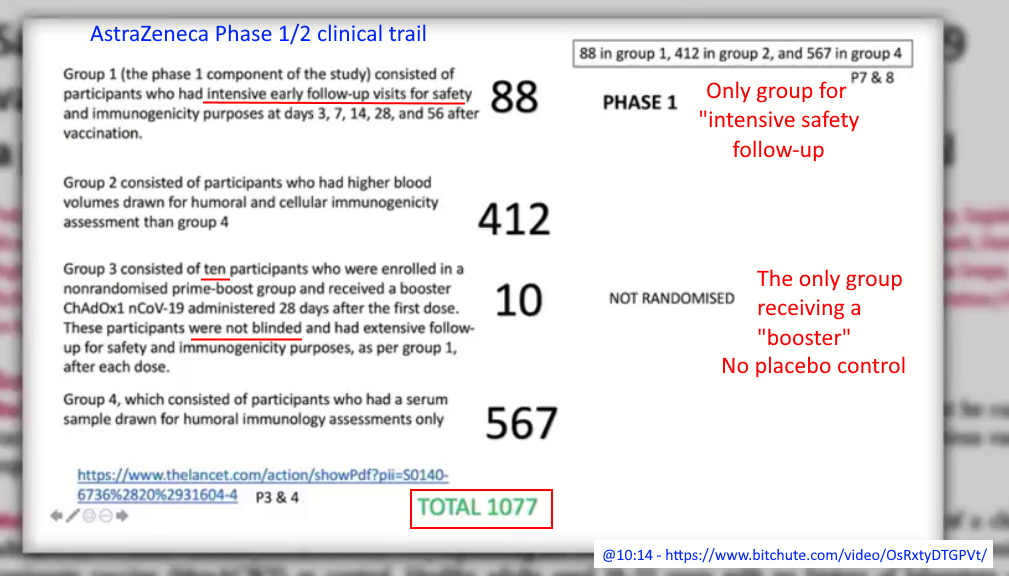
- The “prime-boost group” are the only ones to get two doses which is only 10 people, who are selected with not control group -“All ten participants in the prime-boost group received their booster vaccine at day 28” The remaining 1067 did not receive a booster!
- Of the 10 people CHOSEN for the “booster” group their initial (day zero) T-Cell immunity levels were overall higher that the “control” and treatment participants. Were they hand selected?
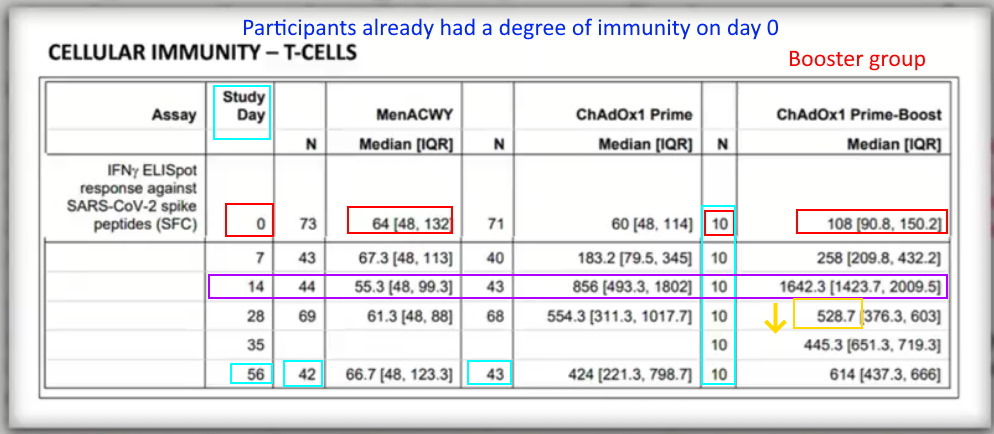
- “However, a boost in cellular responses was not observed following the second ChAdOx1 nCoV-19 dose. This is consistent with previous findings on viral vectored vaccines given as part of a homologous prime-boost regimen.”
- The body develops immunity to the carrier “adenovirus” which is supremely problematic if you want it to be a delivery system for a genetic code.
- Yet this Phase 1/2 AstraZeneca clinical trial, concludes: “Neutralising antibodies were induced in all participants after a second vaccine dose. After two doses, potent cellular and humoral immunogenicity was present in all [TEN] participants studied.” Yet their data show the second dose was only compared at 56 days, and no other comparative data point was provided, but the upper IQR range went higher for the one dose compared to boosted, 798.7 vs 666 respectively.
July 30, 2020 – Reuters: AstraZeneca to be exempt from coronavirus vaccine liability claims in most countries – READ, The Highwire @1:56:20 – WATCH
- “This is a unique situation where we as a company simply cannot take the risk if in … four years the vaccine is showing side effects…”
July 21, 2020 – CBS News: Oxford coronavirus vaccine trial results “extremely encouraging,” U.K. government says – WATCH & READ
- “The results of the Phase I/II trial of the vaccine being developed by Oxford’s Jenner Institute, in conjunction with pharmaceutical giant AstraZeneca, showed that it is safe and “produced strong immune results,” according to … The Lancet medical journal.”
- The vaccine caused a 2-pronged immune response… First, within 14 days, it triggered a T cell response, generating white blood cells that can attack infected cells. Second, within 28 days, it provoked an antibody response. Antibodies are able to prevent the virus from infecting cells when it is initially contracted“
- “Those are two parts of the immune system that ideally work together to protect against viral infections,” Professor Sarah Gilbert,
July 20, 2020 – PHASE 1&2 CLINICAL TRIAL: The Lancet: Safety and immunogenicity of the ChAdOx1 nCoV-19 vaccine against SARS-CoV-2: a preliminary report of a phase 1/2, single-blind, randomised controlled trial by Folegatti et al – – READ, ARCHIVE
July 20, 2020 – Vaccitech Press – Clinical trial data shows Oxford University’s COVID-19 vaccine produces strong immune response – ARCHIVE
- “The results of the Phase I/II trial published today in the scientific journal, The Lancet, indicate no early safety concerns and the induction of strong immune responses in both parts of the immune system.
- The vaccine provoked a T cell response within 14 days of vaccination (a cellular immune response, it could find and attack cells infected with the SARS-CoV-2 virus), and an antibody response within 28 days (humoral immune response, it could find and attack the virus when it was circulating in the blood or lymphatic system).
- “During the study, participants who received the vaccine had detectable neutralising antibodies, which have been suggested by researchers as important for protection, and these responses were strongest after a booster dose, with 100% of participants’ blood having neutralising activity against the coronavirus. The next step in studying the vaccine is to confirm that it can effectively protect against SARS-CoV-2 infection.” [booster here means 2nd dose]
June 15, 2020: Fierce Pharma: AstraZeneca taps Catalent for COVID-19 vaccine finishing, packaging at Italian plant – READ
June 15, 2020 – Reuters: EU governments to pay 750 mln euros for 300 mln doses of AstraZeneca vaccine -Italy – READ
June 11, 2020 – Fierce Pharma: AstraZeneca, Emergent BioSolutions sign $87M deal to produce U.S. supply of COVID-19 vaccine – READ, DEAL
June 10, 2020 – Fierce Pharma: Moderna, AstraZeneca and J&J coronavirus shots rev up for NIH tests beginning in July: WSJ – READ
June 5, 2020 – Fierce Pharma: After Operation Warp Speed picks 5 finalists, experts question why some vaccines were left out – READ – New York Times: Trump Administration Selects Five Coronavirus Vaccine Candidates as Finalists – READ
May 26, 2020 – ClinicalTrials.gov: Investigating a Vaccine Against COVID-19 | Sponsor: University of Oxford – READ
A phase 2/3 study to determine the efficacy, safety and immunogenicity of the candidate Coronavirus Disease (COVID-19) vaccine ChAdOx1 nCoV-19 in healthy UK volunteers
- No placebo control, instead the trial uses a Meningococcal vaccine “MenACWY vaccine”
May 22, 2020 – Science: Doubts greet $1.2 billion bet by United States on a coronavirus vaccine by October – Operation Warp Speed’s funding of AstraZeneca is intended to deliver a COVID-19 vaccine by October, although some call that timeline unrealistic – READ
May 22, 2020 – PRESS RELEASE: Oxford COVID-19 vaccine to begin phase II/III human trials – to enrol up to 10,260 adults and children across UK – READ
May 21, 2020 – New York Times: $1.2 Billion From U.S. to Drugmaker to Pursue Coronavirus Vaccine – The Trump administration [HHS] announced a grant to AstraZeneca, which has licensed a potential vaccine that is in trials by Oxford University. – READ
- The deal with AstraZeneca is the 4th and so far the largest vaccine research agreement that the HHS has disclosed, it is to pay for Phase 3 clinical trial of a potential vaccine in the United States with about 30,000 volunteers.
- Adrian Hill, head the Oxford University Jenner Institute and runs the vaccine project with Sarah Gilbert. “Hill worries about a repeat of his experience in West Africa in 2016, when the disappearance of Ebola ended [Nov 29, 2015] an attempt to test a vaccine his group had designed for that disease.” …”we’re beginning to run out of good trial sites to do vaccine efficacy studies—even the U.S. is plateauing“” Hill said”
May 21, 2020 – A/Z PRESS RELEASE – AstraZeneca advances response to global COVID-19 challenge as it receives first commitments for Oxford’s potential new vaccine – READ
- Company working on a number of agreements in parallel to ensure broad and equitable supply of the vaccine throughout the world at no profit during the pandemic – CEPI, GAVI, WHO
- First agreements to supply at least 400 million doses; Company has total capacity sourced for one billion doses through 2020 and into 2021; continues to increase capacity further
- A/Z received > $1bn from US Biomedical Advanced Research and Development Authority (BARDA) to support development and production of the vaccine including a Phase III clinical trial with 30,000 participants and a paediatric trial.
- AstraZeneca has now finalised its licence agreement with Oxford University for the recombinant adenovirus vaccine. The licensing of the vaccine, formerly ChAdOx1 nCoV-19 and now known as AZD1222
- ChAdOx1 nCoV-19 was developed by Oxford University’s Jenner Institute, working with the Oxford Vaccine Group (Adrian Hill, head the Jenner Institute and runs the vaccine project with Sarah Gilbert)
- A Phase I/II clinical trial of AZD1222 began last month to assess safety, immunogenicity and efficacy in over 1,000 healthy volunteers aged 18 to 55 years across several trial centres in southern England.
May 18, 2020 – Telegraph: Doubts over Oxford vaccine as it fails to stop coronavirus in animal trials – Experts warn that vaccine may only be ‘partially effective’ after results of a trial in rhesus macaque monkeys – READ, Monkeys produced antibodies – READ, but then when reinfected they contracted the disease.
May 16, 2020 – Forbes: Did The Oxford Covid Vaccine Work In Monkeys? Not Really by William Haseltine – READ, The Highwire – WATCH, Published paper – HERE
- “All of the vaccinated monkeys treated with the Oxford vaccine became infected when challenged…there was no difference in the amount of viral RNA detected from this site in the vaccinated monkeys as compared to the unvaccinated animals.” Meaning the vaccinated monkeys, with viable virus in their nose could continue to transmit the virus and infect others. –
- [i.e. the vaccinated and the unvaccinated carried the same viral load, the vaccinated monkeys could infect others, the vaccinated monkeys would test positive for the virus! – REMEMBER THIS!!!]
- “The titer of neutralizing antibody.. is extremely low. Typically, neutralizing antibodies in effective vaccines can be diluted by more than a thousand fold and retain activity. In these experiments the serum could be diluted only by 4 to 40 fold before neutralizing activity was lost.”
- “The authors present evidence to the effect that, although the vaccine did not protect the animals from infection, it did moderate the disease.” But “3 of the 6 vaccinated monkeys were clinically ill” .
- “[N]o evidence of vaccine induced disease enhancement was observed…experience with other vaccines tells us that is not a firm guarantee that such will be the case for humans.”
- “It is crystal clear that the vaccine did not provide sterilizing immunity to the virus challenge, the gold standard for any vaccine. It may provide partial protection.”

May 13, 2020 – Pre-Print: ChAdOx1 nCoV-19 vaccination prevents SARS-CoV-2 1 pneumonia in rhesus macaques – Doremalen, Lambe & Gilbert et al (Oxford and NIH/NIAID researchers) – READ, PDF – Published in Nature July 30, 2020 – READ
April 30, 2020 – A/Z PRESS RELEASE: AstraZeneca and Oxford University announce landmark agreement for COVID-19 vaccine – READ
- This agreement is for the global development and distribution of the University’s potential recombinant adenovirus vaccine aimed at preventing COVID-19 infection from SARS-CoV-2….the vaccine known as ChAdOx1 nCoV-19.
- The potential vaccine entered Phase I clinical trials last week to study safety and efficacy in healthy volunteers aged 18 to 55 years, across five trial centres in Southern England.
- The recombinant adenovirus vector (ChAdOx1) was chosen to generate a strong immune response from a single dose and it is not replicating
- Vaccines made from the ChAdOx1 virus have been given to more than 320 people to date and have been shown to be safe and well tolerated, although they can cause temporary side effects such as a temperature, flu-like symptoms, headache or sore arm.
April 30, 2020 – Viccitech Press: Vaccitech and Oxford University announce landmark partnership with AstraZeneca for the development and large-scale distribution of the COVID-19 vaccine candidate – ARCHIVE
April 27, 2020 – Business Insider: The world’s largest vaccine maker is producing 40 million units of a coronavirus vaccine on trial in Oxford, without yet knowing if it works – READ [with such a huge investment, no wonder they ignored the failed monkey trial results on May 16, 2020!!]
April 27, 2020 – NY Times: In Race for a Coronavirus Vaccine, an Oxford Group Leaps Ahead – As scientists at the Jenner Institute prepare for mass clinical trials, new tests show their vaccine to be effective in monkeys. – READ
April 23, 2020 – SCIENCE: “The Oxford team launched a clinical trial of the vaccine, which contains a harmless chimpanzee adenovirus “vector” carrying the gene for the SARS-CoV-2 surface protein, in 1100 people in the United Kingdom on 23 April. – REF [On April 23, 2020, competitor Sinovac announced it’s monkey trial efficacy results.]
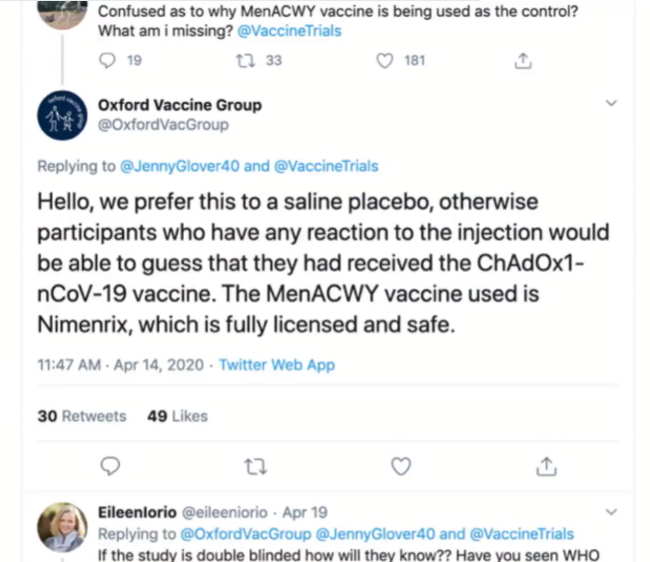
April 14, 2020 – The Oxford vaccine group’s twitter response to why they are NOT using an inert placebo for their control group in their COVID-19 vaccine clinical trials! B/c the meningococcal vaccine is “safe” and will exert the same pain as is expected from the trial vaccine! – The Jaxen Report- SOURCE
- This will hide adverse events!
April 6, 2020 – ClinicalTrial.gov – The University of Oxford changed their clinical trial from a saline placebo to now be “MenACWY Placebo – Standard single dose of MenACWY vaccine delivered intramuscularly” ARCHIVES, CREDIT
- A Study of a Candidate COVID-19 Vaccine (COV001) – NCT04324606 – READ, “last update posted” March 27, 2020 “saline placebo” – ARCHIVE “April 6, 2020″Last Update Posted : April 6, 2020” “Biological: MenACWY Placebo” – ARCHIVE
March 27, 2020 – University of Oxford: COVID-19 vaccine programme starts recruiting for clinical trials- Participants’ screening for the Phase I trial has begun, although the vaccine won’t be ready for a few more weeks. – WATCH, ARCHIVE, TWEET , The Jenner Institute trial recruitment – ANNOUNCEMENT, ARCHIVE, Oxford Vaccine Group recruitment – ARCHIVE, WEB
March 27, 2020 – ClinicalTrials.gov: A Study of a Candidate COVID-19 Vaccine (COV001) – NCT04324606 – Sponsored by University of Oxford – ARCHIVE, READ
- A Phase I/II Study to Determine Efficacy, Safety and Immunogenicity of the Candidate Coronavirus Disease (COVID-19) Vaccine ChAdOx1 nCoV-19 in UK Healthy Adult Volunteers – REF
- A phase I/II single-blinded, randomised, [saline] placebo controlled, multi-centre study to determine efficacy, safety and immunogenicity of the candidate Coronavirus Disease (COVID-19) vaccine ChAdOx1 nCoV-19 in UK healthy adult volunteers aged 18-55 years. The vaccine will be administered intramuscularly (IM)
- Saline placebo gets changed to a “biological” i.e. an active MenACWY vaccine – “Last Update Posted : April 6, 2020” – ARCHIVE
February 13, 2020 – Oxford Science Blog: Outbreak: fighting coronavirus – READ, ARCHIVE
February 4, 2020 – Sasha Latypova Substack (via Feb 2024): Audio recording leaked from AstraZeneca: Covid was classified a national security threat by the US Government/DOD on February 4, 2020, with CEO of AstraZeneca, Pascal Soirot – READ & LISTEN
- “AstraZeneca and other pharma companies participating in the DOD Pandemic Preparedness consortium received a phone call from the DOD saying that “novel covid virus posed national security threat”. This explains why PREP Act declaration in the US was made retroactive to Feb 4, 2020″
- US Department of Defence telling Oxford/AZ to stop working on influenza vaccine and work on the novel coronavirus
2019
March 4, 2019 – Oxford press: Vaccitech Awarded BARDA contract valued at $8.5M to conduct Phase II trial for Influenza Vaccine – READ, ARCHIVE
- Vaccitech’s MVA-M1+NP vaccine candidate aims to prevent pandemic and seasonal influenza by eliciting broader and more durable immune protection against all “A strains” of the virus, which cannot be achieved by traditional vaccination.
January 11, 2019 – Biospace News: Amidst Restructuring and Executives Changes, AstraZeneca Lays off 210 in Colorado – “the company is consolidating “the biologics manufacturing network in one large-scale drug substance facility” in Frederick, Maryland.” – READ
2018
December 20, 2018 – Vaccitech: Vaccitech, a clinical stage developer of a universal flu vaccine and other vaccine-related products, has received a joint £6m investment from a Korean biotech company, GeneMatrix, and venture capital and private equity firm, Korea Investment Partners.- ARCHIVE
November 9, 2018 – Oxford Vaccine Group: How does herd immunity work? – WATCH, SOURCE
- “If the vaccine stops you from catching the disease, you can’t pass it onto anyone else this means that when enough people in the population are protected by vaccination it effectively stops diseases from circulating at all. This is often called herd immunity, but a better name is herd protection, because it helps to protect those who are especially vulnerable to infectious diseases.” WATCH – [well all the COVID-19 vaccines failed this 2018 definition!]
September 29, 2018 -Vaccitech: CEPI Awards Contract Worth Up To USD$19 million to Oxford University and Janssen Vaccines to Develop MERS, Lassa, and Nipah Vaccines – ARCHIVE
September 14, 2018 – New UK-China biotech collaboration to develop shingles vaccine – ARCHIVE
- China’s CanSino Biologics Inc and Vaccitech Ltd. “have signed a Master Collaboration Agreement and initiated their first co-development project under that agreement”
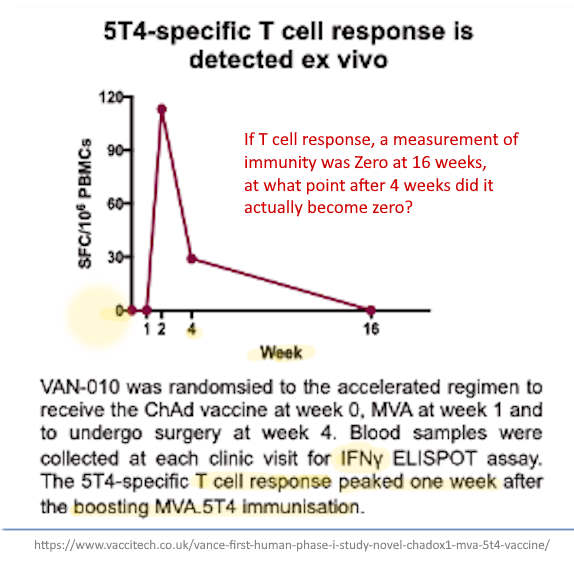
June 5, 2018 – Vaccitech: VANCE: First in Human Phase I Study of a Novel ChAdOx1-MVA 5T4 Vaccine – prostrate cancer – ARCHIVE,
- “Evaluated a novel vaccination platform based on two replication-deficient viruses, chimpanzee adenovirus ChAdOx1 and MVA …” [Vaccinia i.e.cowpox]
- Study funded by European Union Seventh Framework Programm under grant Agreement number 602705 (Project IMPROVE)
March 20, 2018 – Vaccitech: Halts INVICTUS flu study – ARCHIVE
- Primary Care Clinical Trials Unit, Oxford University (PC-CTU) and “Vaccitech has taken the decision not to proceed with season 2 of the current FLU007 (Invictus) study due to the changes recommended by the UK Government which directed the NHS to use FLUAD (adjuvanted TIV) vaccine in the 65 year old and over population in the 2018/19 seasonal influenza vaccination programme. The current FLU007 protocol recommends the use of licensed QIV alongside the Vaccitech vaccine, MVA-NP+M1 in this same age group.”
- [A year later BARDA awards them $8.5M to continue with “MVA-M1+NP vaccine candidate”! – READ]
2017
October 2017 – Vaccitech.co.uk – website first ARCHIVE (scroll down page), CREDIT, Today Vaccitech is Barinthus Biotherapeutics – WIKI
- The founders of the company are Professor Adrian Hill (Director of the Jenner Institute) and Professor Sarah Gilbert (Lead in influenza and MERS),…Vaccitech is engaged in Phase 2 clinical programs for universal influenza vaccine
- Dr Steve Chatfield joins Vaccitech Ltd as Non-Executive Director announced July 21, 2016, formally of Emergent BioSolutions, He began his career working in Vaccine research and development at the Wellcome Foundation, He also serves on the Scientific Advisory Board for the Jenner Institute (Oxford University)… – ARCHIVE
- “Traditional vaccine technology creates a pronounced antibody response to the infectious agent. Vaccitech aim to induce potent and durable antibody responses from the humoral immune system”” “…”Utilizing the world’s leading T cell-inducing platform” – REF
- Investor partners – Emergent BioSolutions – REF, Vaccitech is backed by leading investment institutions, including Sequoia Capital China, Liontrust (formerly Neptune), Korea Investment Partners and Oxford Sciences Innovation and GV (formerly Google Ventures, GV is the venture capital arm of Alphabet, Inc. who owns YouTube, who deletes competing videos!) – ARCHIVE
2016
August 26, 2016 – Vaccitech: OBN Awards 2016: Vaccitech Announced as Finalists – ARCHIVE
- OBN Ltd, the membership organisation supporting the UK’s innovative life sciences companies has announced the finalists of its 8th Annual Awards covering Biotech, Medtech, Synthetic Biology and Digital Health….Vaccitech Limited feature as a finalist for Best Start-up Biotech Company
2003
November 19, 2003 – Intravenous administration of replication-incompetent adenovirus to rhesus monkeys induces thrombocytopenia by increasing in vivo platelet clearance – READ
“…clinical application of these vectors has been hampered by myriad pathologies including fatal multisystem organ failure … Acute thrombocytopenia has been consistently reported in animals and humans.”