PCR testing was used around the world to “diagnose” the disease COVID-19 in anyone, whether they had symptoms or not. It was meant to determine if they were infected with the SARS-CoV-2 virus and thus were potentially “infectious” and able to spread the virus it to others. A positive diagnosis created a “case” of COVID-19. The case statistics were used to show how wide spread the disease was, or how much the virus (SARS-CoV-2) had spread.
A case of COVID-19 “positive” did not mean the person was sick or infections (or even had the virus!). Before the pandemic, a test was normally performed on someone to confirm a diagnosis that a doctor had drawn form existing symptoms – at diagnostic test normally came AFTER symptoms. During the pandemic PCR testing, with amplifications dialled up over 35 cycles up to 45 cycles, created a “casedemic” of false positive statistically ill persons, which these daily advertised chart used to scare the uninformed populations of the world.
This page will collect links to article, papers and videos piecing together the PCR story, from those who were trying to get the message out about it’s misuse, as well as what public health officials were communicating. It will never be complete but is an attempt to capture and organise this historical information.
- PCR specific data points pulled from the COVID-19 Pandemic timeline – HERE
Some background on Polymerase Chain Reaction (PCR)
Not a “test” but a lab technique
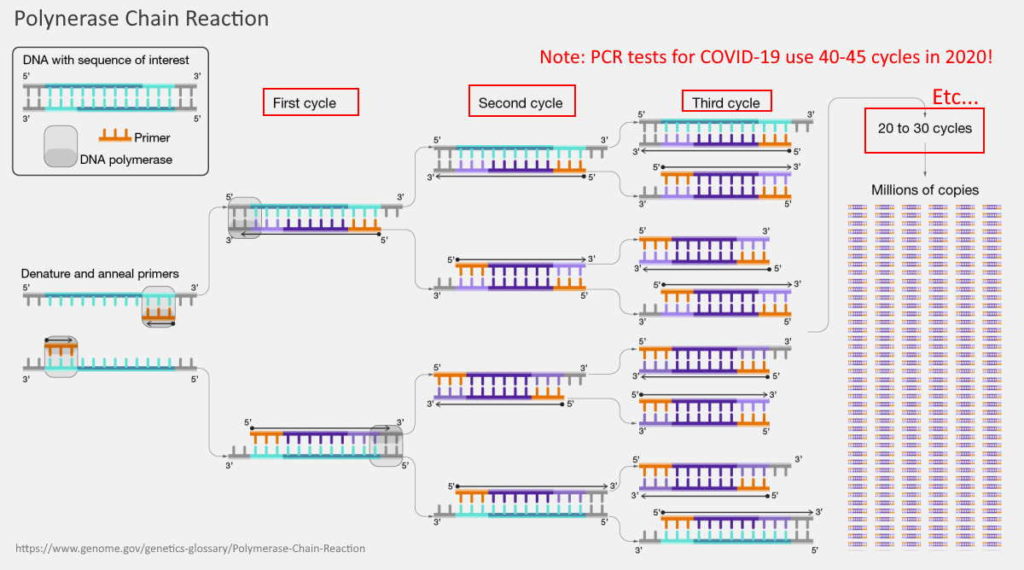
In October 2004 the National Institute of Health Genome division defined polymerase chain reaction (PCR) as “A fast, inexpensive technique for making an unlimited number of copies of any piece of DNA. Sometimes called “molecular photocopying”. So not a test, but a lab technique for amplifying genetic material. [1, 2]
PCR redefined as a “diagnostic test”
By 2020 PCR was redefined as a “Diagnostic Test“. A test to diagnosed a disease, including a symptom-less disease (asymptomatic). A “test” that has been adopted globally to diagnose the disease COVID-19, and thus create the “case” statistics.
A test that amplified a small genetic section of a computer “virus” sequence, where the presence of this small segment was said to prove that someone was “diseased”, or “ill” with COVID-19.
The justification for mass testing everyone, stems from the allegedly phenomenon that healthy individuals showing no symptoms (asymptomatic), could be carriers and spread the virus unknowingly (super-spreaders) to everyone including the vulnerable members of the population – no matter how little viral load they may or may not have.
If someone has an intact immune system and/or had recently recovered from the viral infection, any trace of RNA in their nasal passage could produce a positive result with PCR, it is that sensitive! Contamination during nasal swabbing in carparks could also result in a positive result.
As prophylactic treatments and early treatments were taken off the board by authorities, it magnified the health authorities justification for ‘test, test, test’, as they wanted to ensure isolation of asymptomatic individuals, because the vulnerable allegedly had “no protection” until a miracle vaccine could become available.
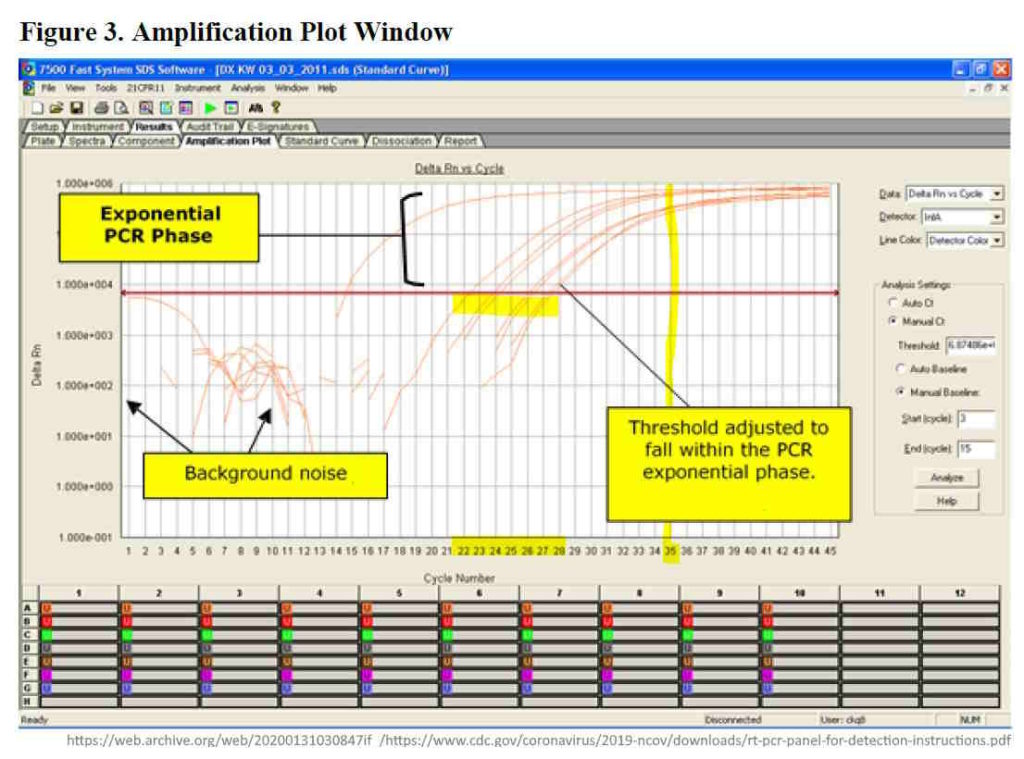
The goal posts relating to “PCR testing” guidelines (i.e. the cycle threshold (Ct) changed after the COVID-19 vaccines rolled out. The higher the cycles above ~28, were highly likely to return a positive PCR result.
Every time the test is cycled, the genetic material doubles in quantity
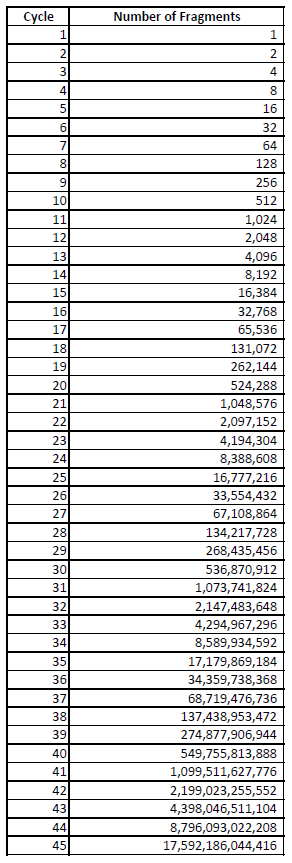
PCR is designed as an laboratory tool to amplify quantities of DNA.
Every time the PCR is ran through a “cycle” the primers pick up complimentary genetic material, if present, and then duplicates- “number of DNA molecules can roughly double in each round of cycling.” There are also primers for fluorescent labelling – so when there is enough quantity present the machine can detect the fluorescence.
There needs to be a certain quantity to register as a fluorescence on the machine. If you cycle enough times, eventually you’ll get a ‘positive’ result for an initial genetic fragment – which would be meaningless.
Table 1 shows a doubling effect, starting with the example of just one “fragment” that contains the genetic sequence you are looking for, and how that quantity can potentially expand with each “cycle”.
Look at the quantity for 28 cycles vs 35 vs 45. Dr Fauci said anything greater than 35 cycles is likely dead nucleotide – thus not infectious, it can’t be cultured! He also states the threshold number (Ct) is not provided with the test result unless you ask for it! Knowledge of the number of cycles use to detect a positive result, can help determine the reliability of that positive result
“…with PCR, if you do it well you can find all most anything in anybody“
Kary Mullis circa 1993
CDC initial PCR instructions (35 then 40 cycles)
On January 24, 2020 the CDC put out instructions for use of RT-PCR, for “the in vitro qualitative detection of 2019-Novel Coronavirus (2019-nCoV) in respiratory specimens and sera.”
Under Interpreting Test Results it states “RP should be positive at or before 35 cycles for all clinical samples and HSC, thus indicating the presence of sufficient nucleic acid from human RNase P gene and that the specimen is of acceptable quality.” – PDF. By February 4, 2020 the PCR threshold was changed to <40 cycles. – PDF
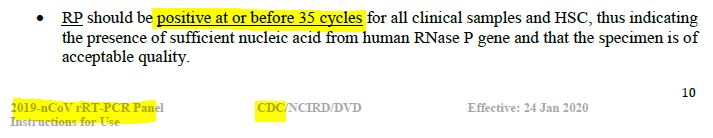
November 2020 – The Highwire Ep 188: COVID TESTING FRAUD UNCOVERED – WATCH
Other consequences of the PCR testing procedure
Before we even get to run the test in the lab, the sample has to be collected. There is a whole host of issues here which I’ll also collect links to, for example:
- Why swab deep into the nasal passage when a mouth swab will suffice?
- The synthetic swabs are sterilised with the ethylene oxide – a known carcinogen
- The synthetic fibre from the swab could sluff-off in the nasal passage
- Cranial injuries occur – for asymptomatic children and adults
- What about that DNA harvesting? Did you give permission?
Other PCR Resource Sites:
- PCR Claims – HERE
- Corman-Drosten Review – ARCHIVE, HERE
- Swiss Policy Research – The Trouble With PCR Tests – HERE
- Health Canada: Polymerase chain reaction (PCR) and cycle threshold (Ct) values in COVID-19 testing – READ, ARCHIVE
PCR related links in reverse chronological order
This content will be updated as time permits
2024
March 16, 2024- Norman Fenton: Helping understand what ‘false positives’ in diagnostic testing really mean – WATCH, READ
- Many still fail to understand the ‘elephant in the room’ – the impact of the underlying population infection rate.
2023
November 13, 2023 – ICAN: Documents Reveal CDC Was Quietly Giving Advice on How to Handle Breakthrough Infections in March 2021 – READ, TIMELINE
- March 2021 – California’s health department alerted the CDC that it was fielding “many calls” for advice on instances of breakthrough infection. i.e.vaccine failure, yet CDC pushed the vaccine worked!
- In May 2021, CDC was given data showing that over 50% of fully vaccinated assisted living facility residents in a California facility tested positive for COVID-19, some of whom were hospitalized or died within just three months of their second dose. i.e. vaccine failure
September 27, 2023 – Australian Bureau of Statistics: COVID-19 first infectious disease in top 5 causes of death since 1970 – READ [REALLY!! – FOIA the death certificates]
- “COVID-19 was the third leading cause of death in 2022, accounting for more than one in 20 deaths (9,859 of 190,939 deaths), according to figures released today by the Australian Bureau of Statistics (ABS).”
- Senator Rennick: “TGA won’t even release the primer sequences used in PCR testing to prove that other strains aren’t being identified as Covid.” – TWEET
- [why in 2022 and not 2020-21?, when we now know the vaccine increases risk of contracting the virus, see June 12, 2023 – HERE]
September 6, 2023 – Epoch Health: People Rarely Transmit COVID-19 Before Experiencing Symptoms: Lancet Study – READ, ARCHIVE, The Lancet – READ
- A blow to the “silent spreader”, super spreader, asymptomatic narrative – which without highly amplified false positive PCR you’d never “know”
- The Lancet: Viral emissions into the air and environment after SARS-CoV-2 human challenge: a phase 1, open label, first-in-human study – Zhou et al – READ, ARCHIVE
- “Very few emissions occurred before the first reported symptom (7%) and hardly any before the first positive lateral flow antigen test (2%),”..
August 4, 2023 – Daily Mail: Thousands of Americans potentially exposed to toxic Covid and pregnancy tests made at Chinese-run biolab in California – READ (not PCR but test kits!)
May 18, 2023 – Norman Fenton Substack | Where are the numbers?: The Covid testing swabs debacle – The ritualistic humiliation and pain was all part of the test of compliance and control – READ

March 16, 2023 – Ann R Coll Surg Engl.: COVID-19 nasopharyngeal swab and cribriform fracture by Vasilica et al – READ, CREDIT
- “non-trivial number” of these swab tests were “associated with skull base injury”
February 24, 2023 – FDA News Release: FDA Authorizes First Over-the-Counter At-Home Test to Detect Both Influenza and COVID-19 Viruses – READ [Not PCR, but “diagnostic” test]
January 30, 2023 – Journal of Neurosurgery” Case Lessons: Cerebrospinal fluid fistula as a complication of reverse transcriptase-polymerase chain reaction collection for the detection of coronavirus disease 2019: illustrative cases – Palavani et al – READ, CREDIT
2022
December 18, 2022 – Health.com: Can Anal Swabs Be Used to Test for Coronavirus? – Nose and throat swabs are standard in the U.S – READ – Timeline of the anal swab research
- April 13, 2022, Stanford Medicine STUDY SARS-CoV-2 infected people can shed viral genetic material in their feces for up to 7 months after diagnosis. “The study added to mounting evidence that the SARS-CoV-2 virus actively infects the gut”
2021
November 14, 2021 – The Telegraph: Covid test firm ‘to sell swabs carrying customers’ DNA’ – Cignpost Diagnostics, a government-approved supplier, prompts investigation over sharing of sensitive medical data with third parties – READ, ARCHIVE, CREDIT
October 28, 2021 – Journal of Surgical Case Reports: Cerebrospinal fluid leak post COVID-19 nasopharyngeal swab for a patient with idiopathic intracranial hypertension: a case report – Asiri et al – READ, CREDIT
July 27, 2021 – Reuters: Fact Check-COVID-19 nasal swabs sterilised with ethylene oxide are safe to use – READ, CREDIT

April 5, 2021 – Very Well Health: New COVID-19 Antibody Test ‘Glows’ When Antibodies Are Present – READ [Antibody not virus but putting here]
- The test, called SATiN (which stands for Serological Assay based on split Tripart Nanoluciferase), uses an enzyme called luciferase (the same enzyme that makes fireflies glow). [Intentional acronym?]
- March 22, 2021- Nature: A homogeneous split-luciferase assay for rapid and sensitive detection of anti-SARS CoV-2 antibodies by Yao et al – READ
- This proof-of-principle study suggests potential applications in diagnostics, as well as disease and vaccination management.
January 27, 2021 – Guardian: China starts using anal swabs to test ‘high-risk’ people for Covid – READ, Reuters – READ, CTV News – READ, Health.com – READ
January 23, 2021 – Peoples Daily | China: Why does nucleic acid testing increase anal swab sampling? Expert Interpretation – (Chinese) READ, CREDIT [Google translator used]
- “Studies have found that the duration of positive nucleic acid in feces or anal swabs of some infected people is longer than that in the upper respiratory tract. Therefore, increasing the nucleic acid detection of anal swabs can increase the detection rate of infected patients and reduce missed diagnoses….At present, anal swab collection is only for key groups.”
- “only throat swabs and anal swabs are collected at the same time for key populations such as isolation points….The purpose of doing so is to increase the detection rate and reduce the probability of false negatives.”
- “In January of 2021, after localized COVID-19 outbreaks in parts of China, including the capital city Beijing, authorities started using anal swabs to test for the virus instead of the usual nose and throat swabs” – REF
DATE? – How the Unscientific Interpretation of RT-PCR & Rapid Antigen Test Results is Causing Misleading Spikes in Cases & Deaths Written By Yohan Tengra & Ambar Koiri for Awaken India Movement (full of references) – PDF
2020
December 17, 2020 – Journal of Clinical Infectious Disease: Predicting Infectious Severe Acute Respiratory Syndrome Coronavirus 2 From Diagnostic Samples – Bullard et al – READ, CEBM – READ, CREDIT
- SARS-CoV-2 Vero cell infectivity of respiratory samples from SARS-CoV-2 positive individuals was only observed for RT-PCR Ct < 24 and symptom onset to test of < 8 days. – REF
- Infectivity of patients with Ct >24 and duration of symptoms >8 days may be low.
December 4, 2020 – Medical Journal of Australia: The COVID swab and the skull base – how to stay safe – Mistry et al – READ
- “The mass testing program during COVID-19 has seen vast numbers of nasal and nasopharyngeal swabs performed. We present an extremely rare case of skull base injury and CSF leak with subsequent meningitis, occurring as a complication of a COVID-19 diagnostic swab.” [rare?]
December 2, 2020 – Peak Prosperity w/ Chris Martensen – The lockdowns are based on surging “cases” which are based on positive PCR test results – explanation of PCR Cycle Thresholds (Ct) – WATCH, BACKUP, BACKUP
November 27, 2020 – Corman Drosten Review: Review report Corman-Drosten et al. Eurosurveillance 2020 – ARCHIVE
- External peer review of the RTPCR test to detect SARS-CoV-2 reveals 10 major scientific flaws at the molecular and methodological level: consequences for false positive results. by Borger, Yeadon, Craig, McKernan et al
November 6 , 2020 – Corona Investigative Committee: Sitzung 26: PCR-Test – die Dominosteine fallen (German) – ARCHIVE
November 5, 2020 – The Highwire Ep 188: Lighting the Way – “covid is over” – FULL, COVID TESTING FRAUD UNCOVERED – WATCH
- Del walks through April 27, 2020 paper by Didier Raoult et al – READ, and shows that a PCR test amplified to 33 cycles returns a false positive 80% of the time, so it is only 20% accurated for the potential presence of viable, infectious virus!
- October 31, 2020 – CNN: US coronavirus cases surpass 9 million driven by ‘silent epidemic’ of asymptomatic infections – READ – As Del Bigtree explains – asymptomatic are not carries, nor are they infections as the US labs set their threshold for cycling the tests at 45 Ct – WATCH
November 2020 (?) – Corman Drosten Review: The consequences of false positives – ARCHIVE
October 31, 2020 – CNN: US coronavirus cases surpass 9 million driven by ‘silent epidemic’ of asymptomatic infections – READ – As Del Bigtree explains – asymptomatic are not carries, nor are they infections as the US labs set their threshold for cycling the tests at 45 Ct – WATCH
October 26, 2020 – from The Highwire: TOP DOC CONFIRMS: COVID IS OVER – featuring Dr Mike Yeadon @MichaelYeadon3 statement Twitter – WATCH , Twitter – ARCHIVE, also on The Highwire – WATCH
I think the PCR test at present is throwing up so many false positves that in fact we’re mis-diagnosing the cause of the deaths that are being reported. The number of deaths that are occurring at the moment is normal for the time of year.
Dr Mike Yeadon
September 29, 2020 – The Lancet Respiratory Medicine | Comment: False-positive COVID-19 results: hidden problems and costs by Surkuva et al – READ, CREDIT
September 28, 2020 – Clinical Infectious Disease | Correspondence: Correlation Between 3790 Quantitative Polymerase Chain Reaction–Positives Samples and Positive Cell Cultures, Including 1941 Severe Acute Respiratory Syndrome Coronavirus 2 Isolates by Faafar et al – READ
- “It can be observed that at Ct = 25, up to 70% of patients remain positive in culture and that at Ct = 30 this value drops to 20%. At Ct = 35, the value we used to report a positive result for PCR, <3% of cultures are positive.”
August 29, 2020 – New York Times: Your coronavirus test is positive. Maybe it shouldn’t be. The usual diagnostic test may simply be too sensitive and too slow to contain the spread of the virus – READ, CREDIT
- “Most tests set the limit at 40, a few at 37 [Ct]…The CDC’s own calculations suggests that it is extremely difficult to detect any live virus in a sample above a threshold of 33 cycles.
- Officials at some state labs said the CDC had not asked them to note threshold values or to share them with contact-tracing organizations” [the logical thing that should have been done to help determine accuracy of test results]
- Thermo Fisher COVID-19 tests “automatically classifies results based on a cut-off of 37 cycles…testers did not have access to the precice numbers”
- “In Massachusetts, from 85 to 90 percent of people who tested positive in July with a cycle threshold of 40 would have been deemed negative if the threshold were 30 cycles, Dr. Mina said. “I would say that none of those people should be contact-traced, not one,”’
- “85 to 90 % of people who tested positive in July 2020 with a cycle threshold of 40 would have been deemed negative if the threshold were 30 cycles, Dr. Mina said. “I would say that none of those people should be contact-traced, not one,”
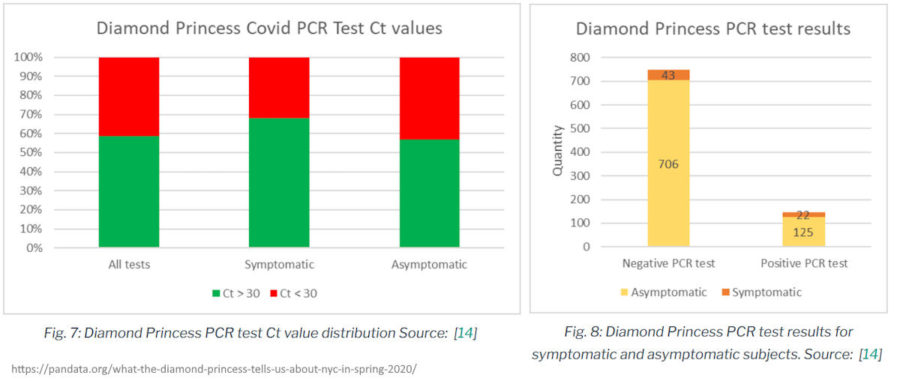
August 18, 2020 – PNAS: Haplotype networks of SARS-CoV-2 infections in the Diamond Princess cruise ship outbreak by Sekizuka et al – READ, PANDA – CREDIT
- The examination of 900 swab samples taken onboard the Diamond Princess passengers and crew February 15 to 17, 2020 “indicates no correlation between Ct value of positive tests, symptomatic vs asymptomatic, or even symptoms vs positive tests. Clearly this sheds some doubt on whether those specimens considered positive really were of live infections, and whether those with symptoms (but testing negative) were actually suffering from Covid-19. In a documentary with footage largely sourced from passenger and crew cell phones, not a single person appeared ill, including some passengers evacuated to hospitals.” PANDA – REF
- 43 people had COVID-19 symptoms, but tested negative, 125 had no symptoms but tested positive!
- The Cq values were not reported for all tests (DATASET 1), of those reported they ranged from 16 to 40, over half were greater than 30 Ct, or the number of cycles the test ran through to get a fluorescent reading.
August 5, 2020 – CEBM: Are you infectious if you have a positive PCR test result for COVID-19? – READ, The Highwire – CREDIT
- At what day of symptom onset did you get the test will influence the probability of a culture positive result.
- SARS-CoV-2 Vero cell infectivity of respiratory samples from SARS-CoV-2 positive individuals was only observed for RT-PCR Ct < 24 and symptom onset to test of < 8 days.
- Most accurate (70-80% cance of true positive result) test day is 3 or 4 days post symptom onset
- Infectivity of patients with Ct >24 and duration of symptoms >8 days may be low.
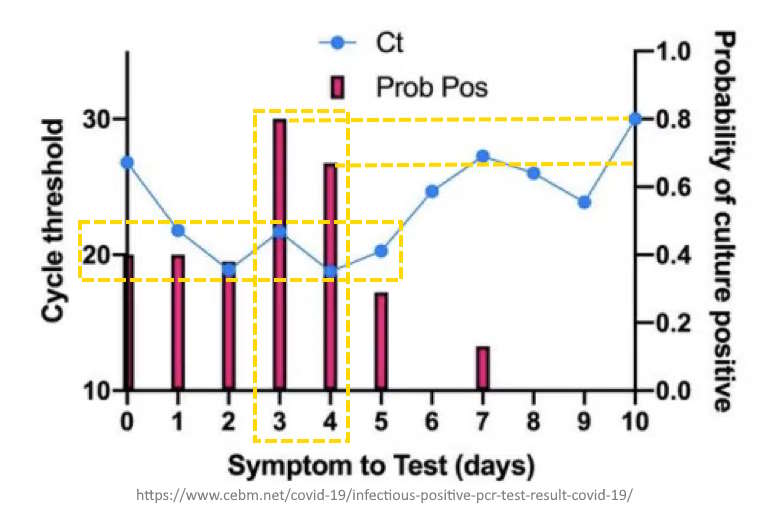
August 4, 2020 – Journal of Clinical Infectious Disease: Predicting Infectious Severe Acute Respiratory Syndrome Coronavirus 2 From Diagnostic Samples – Jefferson et al – READ
July 27, 2020 – The Centre for Evidence-Based Medicine: Predicting infectious SARS-CoV-2 from diagnostic samples- – READ, Bullard et al – READ
Why does the cycle threshold cut-off matter?
- “RT-PCR uses an enzyme called reverse transcriptase to change a specific piece of RNA into a matching piece of DNA. The PCR then amplifies the DNA exponentially, by doubling the number of molecules time and again. A fluorescent signal can be attached to the copies of the DNA, and a test is considered positive when the fluorescent signal is amplified sufficiently to be detectable.
- The cycle threshold (referred to as the Ct value) is the number of amplification cycles required for the fluorescent signal to cross a certain threshold. This allows very small samples of RNA to be amplified and detected.
- The lower the cycle threshold level the greater the amount of RNA (genetic material) in the sample to begin with. The higher the cycle number, the less RNA there is in the sample – the more it needs to be amplified in order to register a flourescent outcome.
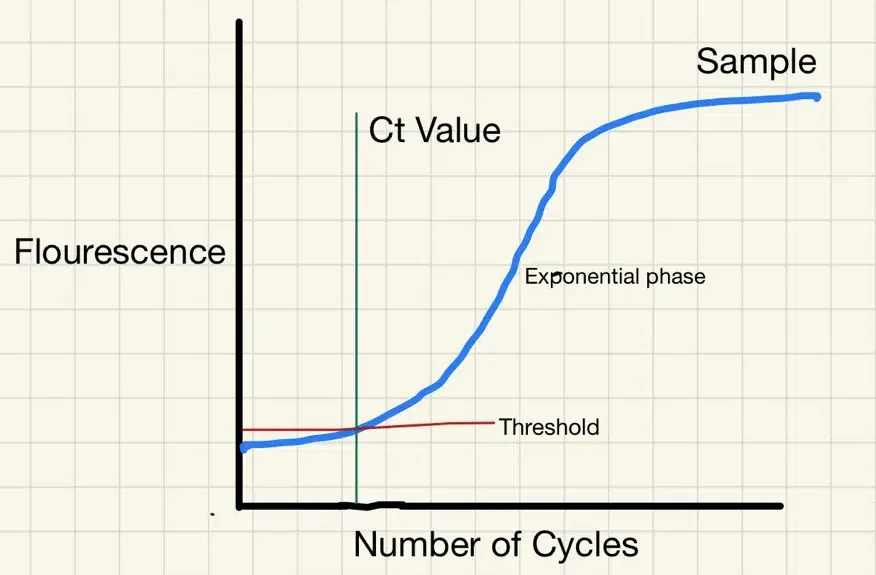
- “…inactivated RNA degrades slowly over time it may still be detected many weeks after infectiousness has dissipated.“
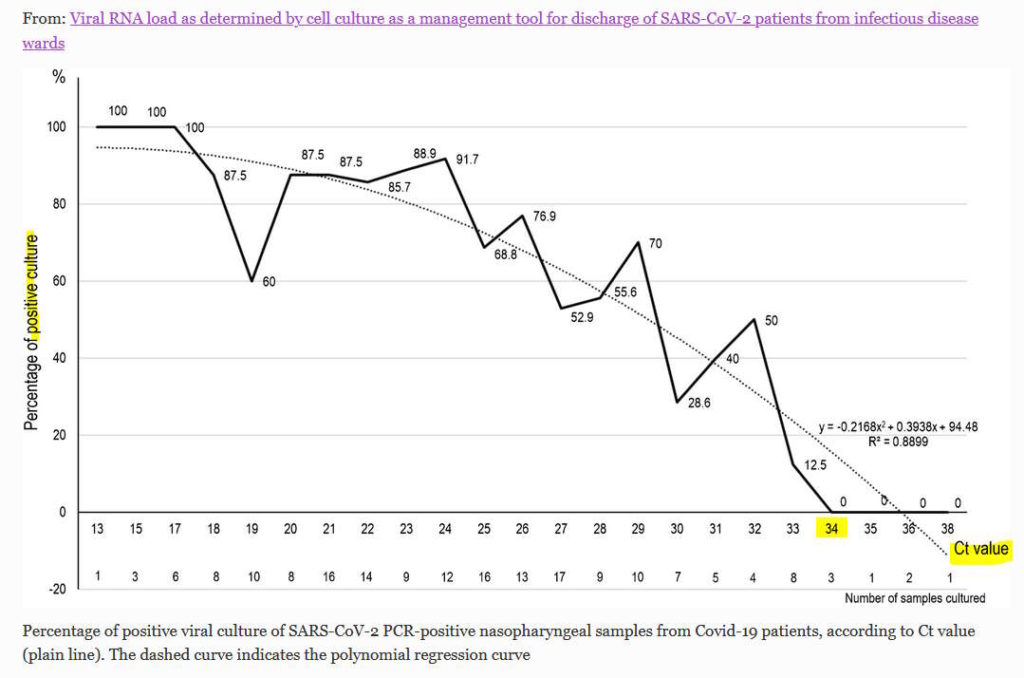
- April 27, 2020 – European Journal of Clinical Microbiology & Infectious Diseases: Viral RNA load as determined by cell culture as a management tool for discharge of SARS-CoV-2 patients for infections disease wards -La Scola, Didier Raoult et al – READ, The Highwire – CREDIT
- SARS-CoV-2 RNA positivity in patient samples was assessed by real-time reverse transcription-PCR targeting the E gene.
- We observed a significant relationship between Ct value and culture positivity rate
- No culture was obtained from samples with Ct > 34
- Our results show that in our system of RT-PCR, we can assess that patients with Ct equal or above 34 may be discharged.
July 16, 2020 – This Week in Virology (TWiV) Ep : Guest Dr Anthony Fauci – WATCH, CREDIT, TIMELINE
July 16, 2020 – This Week in Virology (TWiV) episode 641: COVID-19 with Dr. Anthony Fauci – a discussion on SARS-CoV-2 transmission, testing, immunity, pathogenesis, vaccines, and preparedness – WATCH, READ, PCR 35 cycles – EXCERPT, Ron Paul Liberty Report – EXCERPT, The Highwire – WATCH, TIMELINE
[Re PCR] “What is now sort of evolving into a bit of a standard, that if you get a cycle threshold of 35 or more, that the chances of it being replication competent are minuscule…you almost never can culture virus from a 37 threshold cycle…you gotta say it’s just dead nucleotides, period.”
Dr Fauci – EXCERPT
June 4, 2020 – FDA: Coronavirus (COVID-19) Update: FDA Publicly Shares Antibody Test Performance Data From Kits as Part of Validation Study – READ, CREDIT
May 4, 2020 – Aljazeera: Tanzania president questions coronavirus kits after animal test – President Magufuli says tests were found to be faulty after goat, sheep and pawpaw samples test positive for COVID-19. – READ, ARCHIVE, MSN- ARCHIVE
April 19, 2020 – Nature: Antibody tests suggest that coronavirus infections vastly exceed official counts– Study estimates a more than 50-fold increase in coronavirus infections [CASES] compared to official cases [PCR], but experts have raised concerns about the reliability of antibody kits. – READ
- Maybe becasue it was revealing that COVID-19 could have been through the population months earlier – TIMELINE
April 14, 2020 – The Infectious Myth with David Crowe: Interview with Professor Stephen Bustin on Challenges with RT-PCR – LISTEN, DOWNLOAD, ARCHIVE, David Crown [RIP]: Issues with the RT-PCR Coronavirus Test – CREDIT
- “Professor Bustin stated that cycling more than 35 times was unwise, but it appears that nobody is limiting cycles to 35 or less …Cycling too much could result in false positives as background fluorescence builds up in the PCR reaction.” – REF
- “Implicit in using a Ct number is the assumption that approximately the same amount of original RNA (within a multiple of two) will produce the same Ct number. However, there are many possibilities for error in RT-PCR. There are inefficiencies in extracting the RNA, even larger inefficiencies in converting the RNA to complementary DNA (Bustin noted that efficiency is rarely over 50% and can easily vary by a factor of 10), and inefficiencies in the PCR process itself. Bustin, in the podcast, described reliance on an arbitrary Ct number as “absolute nonsense, it makes no sense whatsoever”. It certainly cannot be assumed that the same Ct number on tests done at different laboratories indicates the same original quantity of RNA.” – REF
- “The Ct cycle number will significantly influence the number of positive tests. If the Ct was changed from 37 to 35 there would be fewer positive tests, and if changed to 39 there would more positive tests.” – [Running all tests up to 45 cycles you create a false positive case-demic!]
- PCR can not distinguish between all stages of a case or no case from colonising virus (infected), naturally immune, infected but clearing dead virus/dead nucleotide, recovered from indection past sympomatic or asymptomatic, meaning the “test” canont diagnose and distiguish between active or defective, full virus particles or broken destroyed virus – it is useless as a stand alone test.
March 16, 2020 – FDA: Coronavirus (COVID-19) Update: FDA Provides More Regulatory Relief During Outbreak, Continues to Help Expedite Availability of Diagnostics – ARCHIVE, CREDIT
- “To facilitate early access to serology tests for laboratories and health care providers, the FDA published guidance on March 16 that permitted developers to market their tests without an EUA as long as the test was validated, the FDA was notified, and test reports included important information about limitations, including statements indicating that the test had not been reviewed by the FDA and that results could not be used to diagnose or exclude infection …By the end of April, 164 commercial manufacturers had notified the FDA that they had introduced serology tests.”- REF
- “In hindsight, however, we realized that the policy outlined in our March 16 guidance was flawed”
- May 4, 2020 – FDA: Insight into FDA’s Revised Policy on Antibody Tests: Prioritizing Access and Accuracy – READ
- Feb 18, 202 – NEJM: The FDA’s Experience with Covid-19 Antibody Tests – READ
March 16, 2020 – WIRED: Everything You Need to Know About Coronavirus Testing- How it works, why we need it, and why it’s taking so damn long for the US to get people diagnosed- ARCHIVE
March 16, 2020 – FDA: Policy for Diagnostic Tests for Coronavirus Disease-2019 during the Public Health Emergency – ARCHIVE
March 16, 2020 – Wired: FDA Approves the First Commercial Coronavirus Tests in the US – Roche RT-PCR tests for COVID-19 and Thermo Fisher for testing instrument – READ
- March 13, 2020 – FDA announcement for ThermoFisher – testing instrument – ARCHIVE, TF – PRESS
- March 16, 2020 – Roche press release – ARCHIVE, not announced by FDA – HERE
March 12, 2020 – AAP News: How to use ICD-10-CM, new lab testing codes for COVID-19 – READ, ARCHIVE
- The Centers for Medicare & Medicaid Services (CMS) developed two new lab testing codes:
- U0001 will be reported for coronavirus testing using the Centers for Disease Control and Prevention (CDC) 2019 Novel Coronavirus Real Time RT-PCR Diagnostic Test Panel
- U0002 will be reported for validated non-CDC laboratory tests for SARS-CoV-2/2019-nCoV (COVID-19)
- Acute respiratory distress syndrome (ARDS): ARDS may develop in conjunction with COVID-19. Cases with ARDS due to COVID-19 should be assigned the codes J80, Acute respiratory distress syndrome, and B97.29
- Do not report “suspected” cases of COVID-19 with B97.29
March 2020 – Luminex SARS-CoV-2 test: Development and Evaluation of Two SARS-CoV-2 RT-PCR Laboratory Developed Tests on the ARIES® Automated, Sample-to-Answer, Real-Time PCR System – Rao et al – PDF,
- This test is “one of the top 4 PCR tests used in the US” – 45 cycles makes it 100% inaccurate if a positive result is returned – The Highwire REF

February 29, 2020 – FDA Update: The FDA encourages development of diagnostic tests for Novel Coronavirus (COVID-19) and we issued an immediately in effect guidance with policy specific to this public health emergency. – ARCHIVE
February 13, 2020 – BBC: Are coronavirus tests flawed? – READ
- “…concerns laboratory tests are incorrectly telling people they are free of the coronavirus!… people are having up to six negative results before finally being diagnosed”. comments from Dr Nathalie MacDermott, of King’s College London
- February 12, 2020 – Radiology: Chest CT for Typical Coronavirus Disease 2019 (COVID-19) Pneumonia: Relationship to Negative RT-PCR Testing – Xie et al –READ
- Some patients with positive chest CT findings may present with negative results for RT PCR for COVID-19
February 4, 2020 – FDA issued an Emergency Use Authorization (EUA) to authorize the emergency use of Centers for Disease Control and Prevention’s (CDC) 2019-Novel Coronavirus (2019-nCoV) Real-Time Reverse Transcriptase (RT)-PCR Diagnostic Panel – ARCHIVE, the same day US HHS Secretary declared a public health emergency
- for the presumptive qualitative detection of nucleic acid from the 2019-nCoV in upper and lower respiratory specimens (such as nasopharyngeal or oropharyngeal swabs, sputum, lower respiratory tract aspirates, bronchoalveolar lavage, and nasopharyngeal wash/aspirate or nasal aspirate) collected from individuals who meet CDC criteria for 2019-nCoV testing…

January 24, 2020: WHO Technical Guidance on SARS-CoV-2 Testing – “Novel Coronavirus (2019-nCoV) technical guidance: Laboratory testing for 2019-nCoV in humans” – ARCHIVE
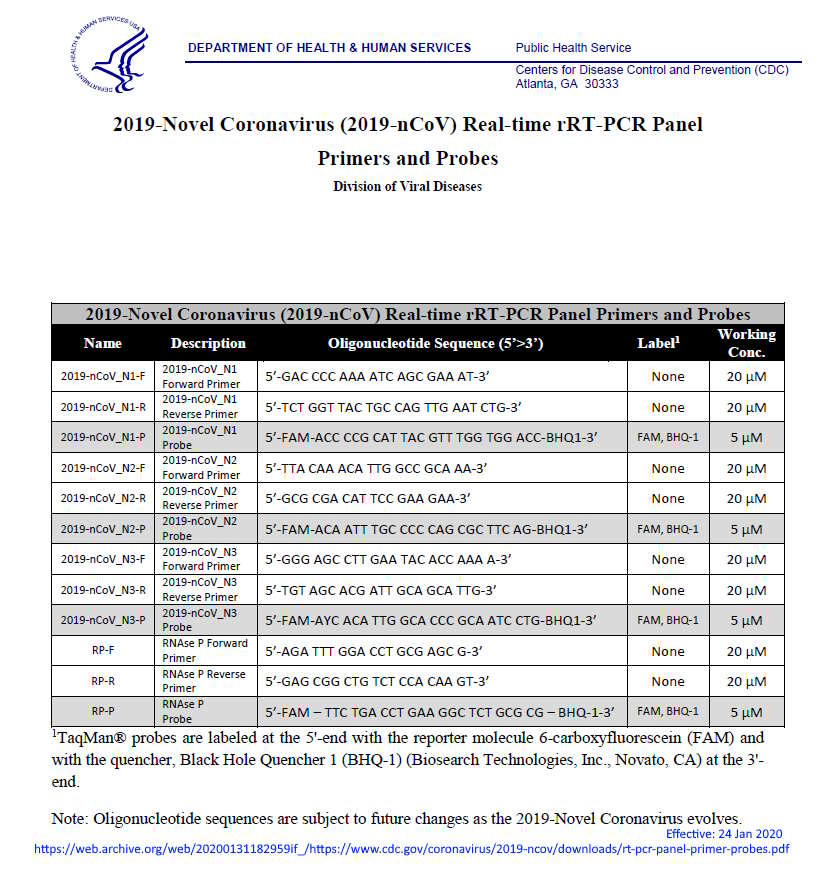
- January 28, 2020 – US CDC: 2019-Novel Coronavirus (2019-nCoV) Real-time rRT-PCR Panel Primers and Probes – ARCHIVE, PDF1, PDF2
- Jan 21, 2020 – China’s Primer and Probes – ARCHIVE
January 24, 2020 – CDC/NCIRD/DVD: Instructions for Use of Real-Time RT-PCR Panel for Detection 2019-Novel Coronavirus – Centers for Disease Control and Prevention (CDC), Respiratory Viruses Branch, Division of Viral Diseases (DVD) – PDF, source ARCHIVE, TIMELINE
- PCR should be positive at or before 35 cycles of amplification. IMAGE. But amplification indicates up to 45 cycles – IMAGE
- 2019-Novel Coronavirus (2019-nCoV) Real-time rRT-PCR Panel Primers and Probes – PDF
- By February 4, 2020 the PCR threshold was changed to <40 cycles. – PDF, IMAGE
January 23, 2020 – Eurosurveillance: Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR – Corman, Drosten et al – READ
January 21, 2020 – National Institute for Viral Disease Control and Prevention CHINA: Specific primers and probes for detection 2019 novel coronavirus Source: Institute of Viral Diseases – ARCHIVE [Google translate used]
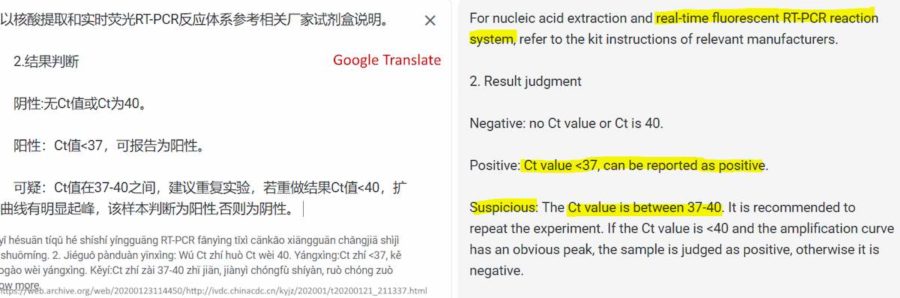
- China Target 1 (ORF1ab):
- Forward primer (F): CCCTGTGGGTTTTACACTTAA
- Reverse primer (R): ACGATTGTGCATCAGCTGA
- Fluorescent probe (P): 5′-FAM-CCGTCTGCGGTATGTGGAAAGGTTATGG-BHQ1-3′
- China Target 2 (N):
- Forward primer (F): GGGGAACTTCTCCTGCTAGAAT
- Reverse primer (R): CAGACATTTTGCTCTCAAGCTG
- Fluorescent probe (P): 5′-FAM-TTGCTGCTGCTTGACAGATT-TAMRA-3
January 17, 2020 – WHO: Laboratory testing for 2019 novel coronavirus (2019-nCoV) in suspected human cases – Interim guidance – ARCHIVE, PDF, SOURCE
January 17, 2020 – WHO: Diagnostic detection of 2019-nCoV by real-time RT-PCR – Protocol and preliminary evaluation – Drosten et al version 2-1- PDF
January 16, 2020 – European Center for Disease Prevention and Control – Laboratory testing of suspect cases of 2019 nCoV using RT-PCR [page now “Case definition for COVID-19” – HERE] – ARCHIVE, SOURCE
January 16, 2020 – Charite Berlin: Erster Test für das neuartige Coronavirus in China entwickelt [Translateed: First test for the novel coronavirus developed detected in China] – READ, CREDIT, Drosten 2017 interview – READ
- “The working group around Prof. Dr. Christian Drosten, Director of the Institute of Virology at Campus Charité Mitte, developed and made available the world’s first diagnostic test as part of her work at the DZIF. The WHO published the test protocol online as the first guide for laboratories. “
- Addendum of March 23, 2020: publication of the test procedure in the journal Eurosurveillance – ARCHIVE
2018
December 28, 2018 – National Cancer Institute at NIH: Cancer-Causing Substances – Ethylene Oxide – (Last updated Dec 28, 2018) – ARCHIVE, [PCR test swabs are soaked in ethylene oxide to sterilise them], 2015 – ARCHIVE, Dec 5, 2022 update LIVE
- “The primary routes of human exposure to ethylene oxide are inhalation and ingestion, which may occur through occupational, consumer, or environmental exposure.”
April 4, 2018 – Technology Networks: The Future for PCR Diagnostics – READ (Article appears to be a promotion for QuantumDx)
- PCR is becoming the preferred method of diagnosis. For example, the method has been endorsed by the World Health Organization (WHO) for rapid diagnosis of pulmonary tuberculosis (TB)2 to replace the standard sputum smear microscopy method developed over a century ago.
- The majority of diagnostics will be based on real-time or quantitative PCR (qPCR). It works by using fluorescently-labeled oligonucleotide probes and monitoring the fluorescence after each cycle – the intensity of the signal reflects the amount of DNA amplified and the number of cycles at which the fluorescence is first detected is used to calculate the initial number of DNA molecules in the sample, once the system has been calibrated.
- amplification of multiple targets in a single PCR experiment by simply using multiple primer pairs…can be useful in diagnosing infectious agents where symptoms are not easily characterized. Up to 20 pathogens can be covered in one test”
- “The potential for PCR diagnostics is huge.” QuantumDx is working on a hand-held PCR device which might one day sit in our home medicine cabinet next to the thermometer
- 2007 Quantum Dx an early stage diagnostic device manufacturer start-up company – “QuantuMDx has successfully completed a proof of concept exercise, identyfying all three Ashkenazi Jewish BRCA mutations from whole blood” – ARCHIVE part of the Personalised and Precision Medicine movement
- 2011 – QuantumDx – ARCHIVE
- 2016 The Future of Diagnostics, hand-held fast diagnostics – ARCHIVE, which integrats diseases diagnosis with the Internet of Things! – ARCHIVE
2017
August 10, 2017 – European J of Clinical Investigation: Talking the talk, but not walking the walk: RT‐qPCR as a paradigm for the lack of reproducibility in molecular research – Stephen Bustin et al – READ, ARCHIVE, David Crowe CREDIT
- “…that the majority of published RT‐qPCR data are likely to represent technical noise”
- April 14, 2020 – Professor Stephen Bustin is a world-renowned expert on quantitative PCR – the Challenges with RT-PCR – LISTEN
2015
April 9, 2015 – Infectious Disease Epidemiology: The History of Pertussis (Whooping Cough); 1906–2015: Facts, Myths, and Misconceptions – Cherry – READ
- “The present “resurgence of pertussis” is mainly due to greater awareness and the use of PCR for diagnosis. There are also many other factors which have contributed to the “resurgence.” New vaccines are clearly needed;…[like mRNA?? because the current “effective” vaccines don’t work?].
- The vaccine stops the cough and allows the infectious agent to spread. “Asymptomatic infections are 4–20 times more common than symptomatic cases”. The vaccinated are a massive sink for spreading B. pertussis!
2008
May 26-27, 2008 – First International Medical Device Regulatory and Compliance Congress held in France – ARCHIVE, ARCHIVE
March 31, 2008- TriMark Publications: Personalized Medicine through Genomic Analysis and Molecular Diagnostics Conference – a critical examination of the molecular diagnostics industry – READ,
- Inspiring a new industry of personalised diagnostic PCR kits and more accessible genetic sequencing such as QuantumDx HERE, HERE, and with use with mobile phone technologies – HERE, CREDIT
2007
October 22, 2007 – TriMark Publications: Point of Care Testing: Innovation on the Move – From Nanotechnology to Personalized Medicine – Conference – READ
January 22, 2007 – Hospital staff at Dartmouth-Hitchcock Medical Center were infomed PCR testing cased a false alarm “pseudo-epidemic” – TIMELINE
2006
March 29-31, 2006 – Harvard holds first annual – Medical Device Regulatory and Compliance Congress – ARCHIVE, ARCHIVE (PCR test is a “medical device”)
- “Benchmarking Your Firm’s Practices in Risk Management, Quality Systems, Fraud and Abuse, and Reimbursement“
- By 2008 – What is FDA’s Focus Today-and its Vision for Tomorrow? – REF
2003
May 22, 2003 – WHO WHA launch the Foundation for Innovative New Diagnostics (FIND) – ARCHIVE
- Because “there is an urgent and unmet need for more accurate and cost-effective diagnostic technologies, particularly for diseases devastating the developing world.”…”Diagnostic tests are also the foundation of disease surveillance and elimination.”
- [So begins the new era of Diagnostics (Dx) – accelerating towards gene-based Personalised Medicine]
- “Diagnosis is the first step on the path to treatment and the foundation of disease control and prevention” – REF
- [With an approved “diagnosis”, an “evidence-basedTM” treatment “protocol” can then applied, thus eventually eliminating the need for a doctor! Diagnosis assumes one causal agent for all dis-ease]
“There are three phases to treatment: diagnosis, diagnosis and diagnosis
Dr William Osler (1892), the father of modern medicine,
1987
1987 – Methods in Enzymology: Specific synthesis of DNA in vitro via a polymerase-catalyzed chain reaction – Kary Mullis and Fred Faloon – READ, CREDIT
1984
March 1, 1984 – Journal Clinical Microbiology: Role of recent vaccination in production of false-positive coronavirus antibody titres in cats – Barlough et al – READ Referenced by Dr Buttar (antibody false positive is different to PCR, but important to note.)